李炜娟,任清风,徐群英,张中伟,李伟,冯建高,任晓慧,肖元梅∆
慢性铅暴露对大鼠脑组织XRCC1 mRNA表达的影响及其与氧化应激的关系
李炜娟1,2,任清风1,3,徐群英1,张中伟1,李伟1,冯建高1,任晓慧1,肖元梅1∆
摘要:目的观察饮水铅暴露对大鼠大脑皮质、小脑、海马组织中X线交错互补修复基因1(XRCC1)mRNA表达的影响及其与氧化应激的关系。方法40只SD大鼠根据体质量按随机区组法分对照组和4个铅暴露组:最低剂量组、低剂量组、中剂量组、高剂量组,对照组自由饮用去离子水,4个铅暴露组分别饮用100、200、400、800 mg/L的醋酸铅溶液,连续染毒60 d后取大脑皮质、小脑和海马。RT-PCR技术检测脑组织XRCC1 mRNA的表达量,并测定脑组织铅、过氧化氢酶(CAT)、谷胱甘肽(GSH)和过氧化氢(H2O2)的含量。结果与对照组比较,铅暴露组大鼠大脑皮质、小脑和海马中XRCC1 mRNA表达量、脑铅的含量和H2O2水平均升高(P<0.05);而CAT、GSH含量基本低于对照组(P < 0.05);相关性分析显示铅暴露组大鼠大脑皮质、小脑和海马中XRCC1 mRNA表达量与脑组织铅含量呈正相关(r分别为0.608、0.438、0.470,P<0.01),与CAT、GSH呈负相关(r分别为-0.343、-0.465、-0.423,-0.383、-0.489、-0.366,P<0.05),与H2O2呈正相关(r分别为0.455、0.517、0.342,P<0.05)。结论铅可通过诱导细胞氧化应激而影响XRCC1 mRNA的表达。
关键词:铅;X线交错互补修复基因1;氧化应激;过氧化氢酶;谷胱甘肽;过氧化氢;脑组织
∆通讯作者E-mail: xym72@163.com
铅是一种广泛存在于环境中的重金属毒物,可造成多系统多器官损伤。神经系统对铅中毒最为敏感,微量铅暴露便可以引起神经系统的功能异常。随着研究的不断深入,氧化应激损伤被认为是铅致神经系统损伤的主要原因[1-2]。铅中毒诱导机体产生大量活性氧自由基,可能导致DNA分子发生氧化损伤。碱基切除修复(base excision repair, BER)是最常见的DNA损伤修复形式,BER过程中,X线交错互补修复基因1(XRCC1)充当脚手架蛋白的作用,其本身不具有酶的活性,主要是通过与多种酶(DNA多聚酶β、PARP1、DNA连接酶Ⅲ等)结合从而参与损伤位点的修复[3-5]。以往研究多关注于XRCC1基因多态性与铅中毒的易感性,而有关铅对XRCC1基因mRNA表达的研究报道少见。本文通过检测大鼠大脑皮质、小脑、海马组织中XRCC1 mRNA的表达水平,分析其与脑组织铅含量及氧化应激指标之间的关系,以期为进一步从分子水平探讨铅的神经毒性机制提供科学依据。
1 材料与方法
1.1主要试剂与仪器乙酸铅购于西陇化工厂;RNA提取试剂盒购于上海索莱宝生物科技公司;引物由Invitrogen公司合成;逆转录试剂盒购于美国Thermo公司;铅标准溶液购于国家标准物质研究中心;过氧化氢酶(CAT)、谷胱甘肽(GSH)和过氧化氢(H2O2)测定试剂盒购于碧云天生物技术研究所。AA-6300C型石墨炉原子吸收分光光度计(日本岛津公司);AAS SOLAAR M6石墨炉原子吸收分光光度计(美国Thermo公司);PCR扩增仪(美国BIO-RAD);多功能酶标仪(美国Molecular Devices);722型可见分光光度计(上海精密科学仪器公司);凝胶成像分析系统(美国Synoptics公司)。
1.2实验动物分组及处理40只刚断乳、雄性、健康SPF级SD大鼠,体质量90~100 g,购自北京维通利华实验动物有限公司,动物合格证号:SCXK(京)2012-0001。适应性喂养1周后,根据体质量按随机区组法分为对照组和4个铅暴露组,每组8只,分别自由饮用去离子水(对照组)、100 mg/L的乙酸铅溶液(最低剂量组)、200 mg/L的乙酸铅溶液(低剂量组)、400 mg/L的乙酸铅溶液(中剂量组)、800 mg/L的乙酸铅溶液(高剂量组),动物染毒和处理方法参照肖元梅等[2]的方法。
1.3检测指标及其方法(1)RT-PCR检测XRCC1 mRNA表达水平:E.Z.N.A.TMDNA/RNA/Protein Isolation Kit试剂盒提取大鼠脑组织RNA,检测RNA纯度及浓度后使用Rever⁃tAid First Strand cDNA Synthesis kit进行逆转录反应(RT),Dream Tap Green PCR Master Mix (2×)进行聚合酶链式扩增反应(PCR)。XRCC1引物,上游5′- AGAGGCTGACCTGC⁃CAATTC-3′,下游5′-CGGTCGCTCATGTAGTCCTC-3′;内参基因β-actin引物,上游5′-CTGTGTGGATTGGTGGCTCT-3′,下游5′-GCTCAGTAACAGTCCGCCTA-3′。程序:95℃2 min;95℃30 s,54℃(β-actin)或58℃(XRCC1)30 s,72℃40 s, 40个循环;72℃7 min。(2)脑组织经消化后测定铅的含量[6]。(3)CAT、GSH和H2O2含量测定参照任清风等[7]的方法。
2 结果
2.1铅暴露对大鼠大脑皮质、小脑、海马中XRCC1 mRNA表达的影响4个铅暴露组大鼠大脑皮质、小脑和海马中的XRCC1 mRNA表达量均高于对照组(P<0.05),而4个染铅剂量组间两两比较均无明显差异(P>0.05),见表1、图1。
Tab. 1 Comparison of the expression of XRCC1 mRNA in cerebral cortex, hippocampus and cerebellum between five groups表1 各组大鼠脑组织中XRCC1 mRNA表达水平比较(n=8,±s)
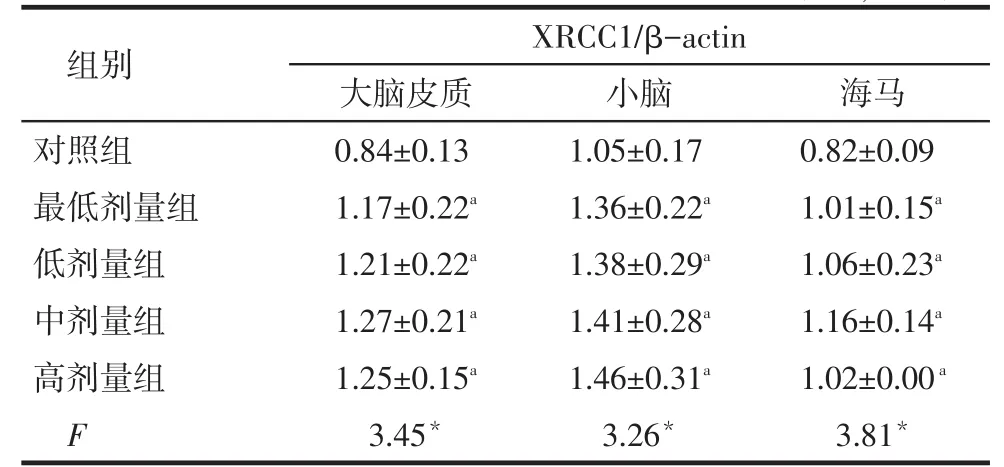
Tab. 1 Comparison of the expression of XRCC1 mRNA in cerebral cortex, hippocampus and cerebellum between five groups表1 各组大鼠脑组织中XRCC1 mRNA表达水平比较(n=8,±s)
*P < 0.05;a与对照组比较,P < 0.05
组别对照组最低剂量组低剂量组中剂量组高剂量组F XRCC1/β-actin大脑皮质0.84±0.13 1.17±0.22a1.21±0.22a1.27±0.21a1.25±0.15a3.45*小脑1.05±0.17 1.36±0.22a1.38±0.29a1.41±0.28a1.46±0.31a3.26*海马0.82±0.09 1.01±0.15a1.06±0.23a1.16±0.14a1.02±0.00a3.81*
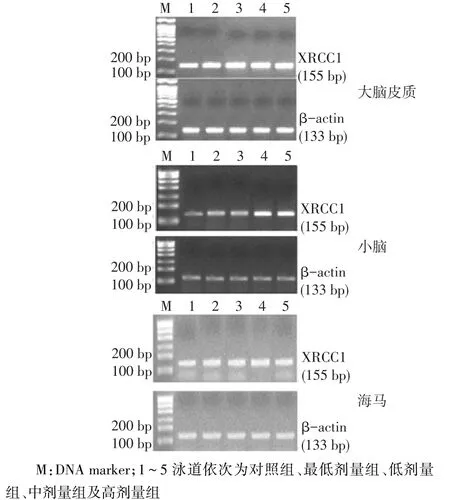
Fig. 1 The expression of XRCC1mRNA in cerebral cortex, hippocampusand cerebellum in five groups图1 各组大鼠大脑皮质、小脑和海马中XRCC1 mRNA表达电泳图
2.2大鼠脑组织中铅含量的变化4个铅暴露组大鼠大脑皮质、小脑、海马铅的含量均高于对照组(P<0.05),且随染铅水平增高,脑组织中的铅水平基本呈同步增高趋势,见表2。
Tab. 2 Comparison of lead levels in brain tissue betweenfive groups表2 各组大鼠脑组织中铅含量比较 (n=8,μg/g,±s)
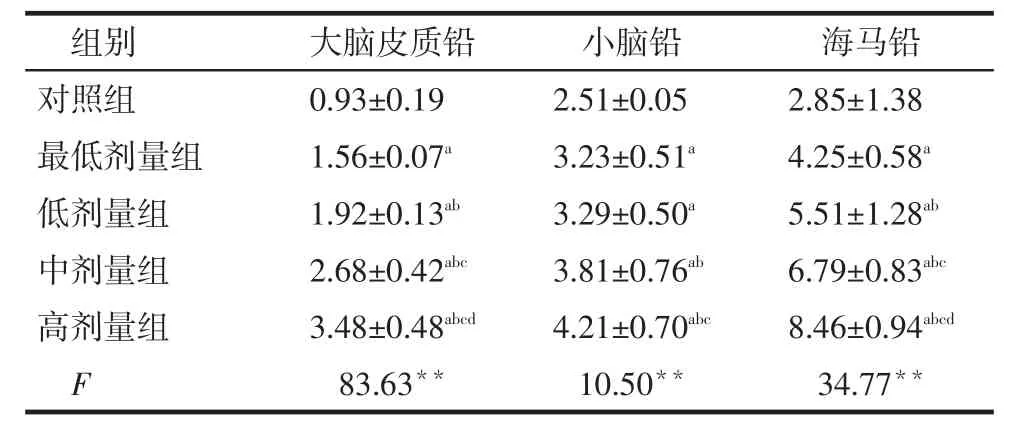
Tab. 2 Comparison of lead levels in brain tissue betweenfive groups表2 各组大鼠脑组织中铅含量比较 (n=8,μg/g,±s)
**P<0.01;a与对照组比较,b与最低剂量组比较,c与低剂量组比较,d与中剂量组比较,P < 0.05
组别对照组最低剂量组低剂量组中剂量组高剂量组F大脑皮质铅0.93±0.19 1.56±0.07a1.92±0.13ab2.68±0.42abc3.48±0.48abcd83.63**小脑铅2.51±0.05 3.23±0.51a3.29±0.50a3.81±0.76ab4.21±0.70abc10.50**海马铅2.85±1.38 4.25±0.58a5.51±1.28ab6.79±0.83abc8.46±0.94abcd34.77**
2.3各组大鼠脑组织CAT、GSH和H2O2水平比较染铅后,大脑皮质、小脑和海马CAT、GSH含量基本低于对照组而H2O2水平高于对照组(P < 0.05),并随着染铅剂量的升高,脑组织CAT含量基本呈逐渐下降趋势,而H2O2水平基本呈逐渐升高趋势,见表3~5。
Tab. 3 Comparison of CAT levels in brain tissue betweenfive groups表3 各组大鼠脑组织CAT水平比较(n=8,U/mg prot,±s)
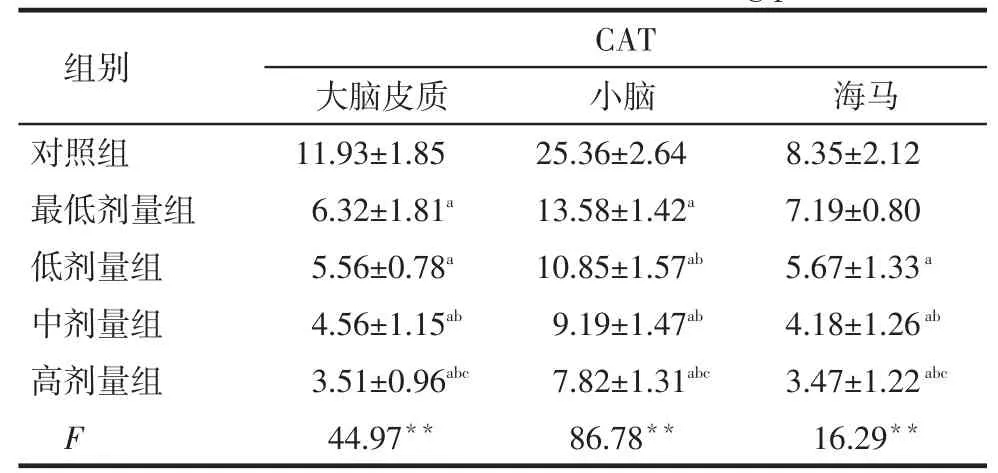
Tab. 3 Comparison of CAT levels in brain tissue betweenfive groups表3 各组大鼠脑组织CAT水平比较(n=8,U/mg prot,±s)
**P<0.01;a与对照组比较,b与最低剂量组比较,c与低剂量组比较, P < 0.05
组别对照组最低剂量组低剂量组中剂量组高剂量组F CAT大脑皮质11.93±1.85 6.32±1.81a5.56±0.78a4.56±1.15ab3.51±0.96abc44.97**小脑25.36±2.64 13.58±1.42a10.85±1.57ab9.19±1.47ab7.82±1.31abc86.78**海马8.35±2.12 7.19±0.80 5.67±1.33a4.18±1.26ab3.47±1.22abc16.29**
Tab. 4 Comparison of GSH levels in brain tissue betweenfive groups表4 各组大鼠脑组织GSH水平比较(n=8,mg/L,±s)
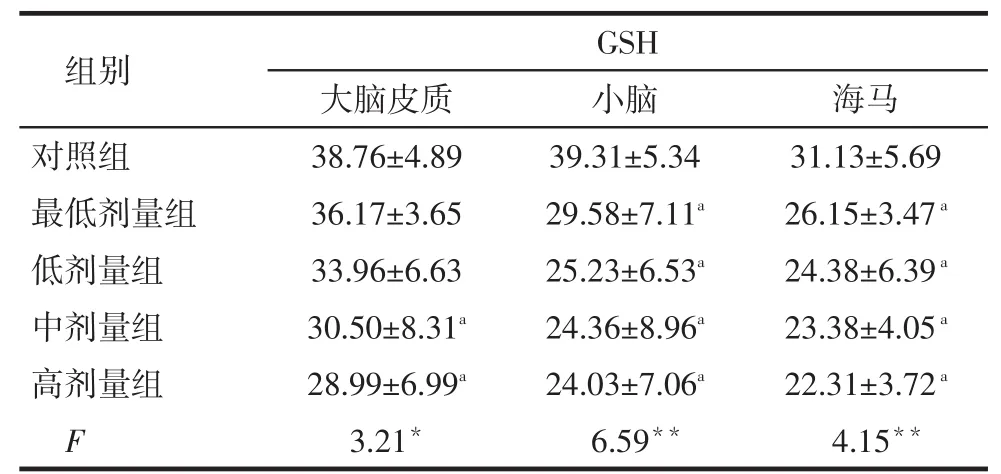
Tab. 4 Comparison of GSH levels in brain tissue betweenfive groups表4 各组大鼠脑组织GSH水平比较(n=8,mg/L,±s)
*P < 0.05,**P<0.01;a与对照组比较,P < 0.05
组别对照组最低剂量组低剂量组中剂量组高剂量组F GSH大脑皮质38.76±4.89 36.17±3.65 33.96±6.63 30.50±8.31a28.99±6.99a3.21*小脑39.31±5.34 29.58±7.11a25.23±6.53a24.36±8.96a24.03±7.06a6.59**海马31.13±5.69 26.15±3.47a24.38±6.39a23.38±4.05a22.31±3.72a4.15**
Tab. 5 Comparison of H2O2levels in brain tissue between five groups表5 各组大鼠脑组织H2O2水平比较(n=8,mmol/g prot,±s)

Tab. 5 Comparison of H2O2levels in brain tissue between five groups表5 各组大鼠脑组织H2O2水平比较(n=8,mmol/g prot,±s)
**P<0.01;a与对照组比较,b与最低剂量组比较,P < 0.05
组别对照组最低剂量组低剂量组中剂量组高剂量组F H2O2大脑皮质16.37±3.33 20.02±2.29 23.17±5.32a25.49±8.99ab26.85±1.29ab5.65**小脑9.20±0.58 14.97±1.71a15.51±1.96a16.44±2.11a17.38±2.53ab22.88**海马11.93±1.31 13.11±2.07 14.69±2.06a15.35±2.96ab16.02±1.34ab5.38**
2.3XRCC1 mRNA表达量与脑组织铅含量和氧化应激指标的相关性大脑皮质、小脑和海马XRCC1 mRNA表达量均与其组织铅、H2O2含量呈正相关,与CAT、GSH含量呈负相关(均P<0.05),见表6。
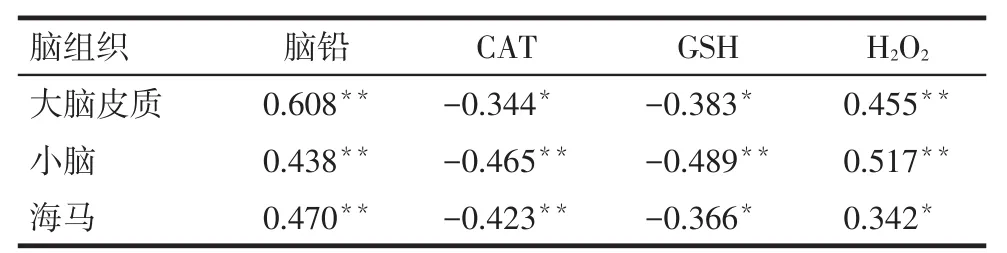
Tab. 6 The correlation analysis of the expression of XRCC1 mRNA, lead levels and oxidative stress indicators in brain tissues表6 XRCC1 mRNA表达量与脑组织铅含量和氧化应激指标的相关性分析 (r)
3 讨论
人类XRCC1是参与修复电离辐射对细胞DNA损害的第一个哺乳动物类基因[8]。XRCC1基因编码的蛋白质在DNA单链断裂修复过程中发挥了重要作用,主要作用于碱基切除修复途径。BER过程中,XRCC1编码的蛋白质充当脚手架蛋白的作用,虽然它本身不具有酶的活性,但是它可以通过与多种酶(如PARP1、DNA连接酶Ⅲ、DNA多聚酶β)结合形成复合物参与DNA损伤位点的修复,保证了DNA修复的精确性,从而发挥了XRCC1在BER过程中的核心地位[3-5,9-10]。
对XRCC1 mRNA表达的研究,不同研究者所得结果不尽相同,甚至相反。Yoo等[11]的体外实验研究表明DNA损伤剂可使XRCC1 mRNA表达下降,并随着DNA损伤程度的增加而下降。Fujimura等[12]研究表明,正常大鼠大脑皮质、小脑、海马都存在XRCC1的表达,主要在细胞核内表达,其中脑组织中海马区域表达量最高,氧化应激损伤时,大脑皮质、小脑、海马损伤区域XRCC1基因mRNA的表达量随着DNA损伤程度的增加而明显减少。而Bus⁃ciglio等[13]研究却显示唐氏综合征患者的颞叶、顶叶和枕叶中XRCC1基因mRNA表达量明显增加,认为表达增加主要是由于活性氧自由基的大量堆积。Fang-Kircher等[14]研究显示唐氏综合征患者额叶和小脑区XRCC1基因mRNA表达量不变甚至是降低。Bosshard等[15]研究显示海人酸(KA)诱导大鼠癫痫样发作16 h后,海马CA1、CA3和海马回等处XRCC1基因mRNA表达上调。本研究显示,铅染毒组大鼠大脑皮质、小脑和海马组织中XRCC1基因mRNA的表达量高于对照组,与文献[11-12,14]研究不同,可能是不同的氧化应激诱导物导致DNA损伤的程度不同,进而导致XRCC1基因mRNA的表达量不同。铅染毒组大鼠脑组织中XRCC1基因mRNA的表达量与组织铅含量呈正相关,与抗氧化指标CAT、GSH含量呈负相关,与氧化指标H2O2呈正相关,表明铅暴露后大鼠脑组织XRCC1基因mRNA表达变化与铅所致脑组织氧化应激损伤密切相关。
综上,慢性铅暴露使大鼠大脑皮质、小脑、海马XRCC1 mRNA表达量增加,且与组织铅含量和H2O2呈正相关,与CAT、GSH呈负相关,说明铅可通过诱导细胞氧化应激损伤而影响XRCC1基因mRNA的表达,但其具体的分子机制有待于进一步深入研究。
参考文献
[1] Bas H, Kalender Y, Pandir D, et al. Effects of lead nitrate and sodium selenite on DNA damage and oxidative stress in diabetic and nondiabetic rat erythrocytes and leucocytes[J]. Environ Toxicol Pharmacol, 2015, 39(3): 1019-1026. doi:10.1016/j.etap.2015.03.012.
[2] Xiao YM, Xu QY, Zhang ZW, et al. Effects of lead exposure by drinking water on hydrogen peroxide, hydroxyl free radicals and lipid peroxi⁃dation in brain tissues of rats [J]. Tianjin Med J, 2015, 43(10): 1119-1121.[肖元梅,徐群英,张中伟,等.饮水铅暴露对大鼠脑组织过氧化氢、羟自由基和脂质过氧化的影响[J].天津医药, 2015, 43(10): 1119-1121]. doi:10.11958/j.issn.0253-9896.2015.10.009.
[3] McNeill DR, Lin PC, Miller MG, et al. XRCC1 haploin sufficiency inmice has little effect on aging, but adversely modifies exposuredependent susceptibility[J]. Nucleic Acids Res, 2011, 39 (18): 7992-8004. doi: 10.1093/nar/gkr280.
[4] Campalans A, Kortulewski T, Amouroux R, et al. Distinct spatiotem⁃ poral patterns and PARP dependence of XRCC1 recruitment to sin⁃gle-strand break and base excision repair[J]. Nucleic Acids Res, 2013, 41(5): 3115-3129. doi: 10.1093/nar/gkt025.
[5] Hanssen-Bauer A, Solvang-Garten K, Sundheim O, et al. XRCC1 coordinates disparate responses and multiprotein repair complexes depending on the nature and context of the DNA damage[J]. Envi⁃ron Mol Mutagen, 2011, 52(8):623-635. doi: 10.1002/em.20663.
[6] Liang XH, Jiao Y, Feng RP, et al. Comparative study of strong acid digestion method and trypsin digestion method for extract diatom [J]. Forensic Science and Technology, 2008, 4:7-9.
[7] Ren QF, Li WJ, Xu QY, et al. Study on the expression of APE1 pro⁃tein and their relationships with oxidative stress in brain tissues of rats induced by lead exposure through drinking water[J]. Tianjin Med J,2016,44(2):170-172. [任清风,李炜娟,徐群英,等.饮水铅暴露对大鼠脑组织APE1表达的影响及与氧化应激的关系研究.天津医药, 2016, 44(2):170-172]. doi:10.11958/20150148.
[8] Hanssen-Bauer A, Solvang-Garten K, Akbari M, et al. X-Ray re⁃pair cross complementing protein 1 in base excision repair[J]. Int J Mol Sci, 2012, 13(12):17210-17229. doi: 10.3390/ijms131217210.
[9] Wang SY, Gong ZH, Chen R, et al. JWA regulates XRCC1 and func⁃tions as a novel base excision repair protein in oxidative-stress-in⁃duced DNA single-strand breaks[J]. Nucleic Acids Res, 2009,37(6): 1936-1950. doi: 10.1093/nar/gkp054.
[10] Li Y, Li SY, Wu ZW, et al. Polymorphisms in genes of APE1, PARP1, and XRCC1: risk and prognosis of colorectal cancer in a northeast Chinese population[J]. Med Oncol, 2013, 30(2): 505-508.
[11] Yoo H, Li L, Sacks PG. Alteration in expression and structure of the DNA repair gene XRCC1[J]. Biochem Biophys Res Commun, 1992, 186(2):900-910.
[12] Fujimura M, Morita-Fujimura Y, Sugawara T, et al. Early decrease of XRCC1, a DNA base excision repotein, may contribute to DNA fragmentation after transient focal cerebral ischemia in mice[J]. Stroke,1999, 30(11):2456-2462.
[13] Busciglio J, Yankner BA. Apoptosis and increased generation of re⁃active oxygen species in Down′s syndrome neurons in vitro[J]. Na⁃ture, 1995, 378(6559):776-779.
[14] Fang- Kircher SG, Labudova O, Kitzmueller E, et al. Increased steady state mRNA levels of DNA-repair genes XRCC1, ERCC2 and ERCC3 in brain of patients with Down syndrome[J]. Life Sci, 1999, 64(18): 1689-1699.
[15] Bosshard ME, Markkanen E, Loon B. Base excision repair in physi⁃ology and pathology of the central nervous system[J]. Int J Mol Sci, 2012, 13(12): 16172-16222.doi: 10.3390/ijms131216172.
2015-09-10收稿2015-10-20修回)
(本文编辑闫娟)
作者单位:1南昌大学公共卫生学院(邮编330006);2南昌大学抚州医学院;3九江学院临床医学院·附属医院
Effects of chronic lead exposure on expression of XRCC1 gene mRNA and its relationships with oxidative stress in brain tissues of rats
LI Weijuan1,2, REN Qingfeng1,3, XU Qunying1, ZHANG Zhongwei1, LI Wei1, FENG Jiangao1, REN Xiaohui1, XIAO Yuanmei1∆
1 School of Public Health, Nanchang University, Nanchang 330006, China; 2 Fuzhou Medical College of Nanchang University; 3 Jiujiang University Clinical Medical College, Jiujiang University Hospital
∆Corresponding Author E-mail: xym72@163.com
Abstract:ObjectiveTo observe the effects of lead exposure through drinking water on the expression of XRCC1 mRNA in cerebral cortex, cerebellum and hippocampus of rats and its relationship with oxidative stress. Methods Forty SD rats were divided randomly into five groups: control group and four exposure groups (100 mg/L, 200 mg/L, 400 mg/L and 800 mg/L lead acetate for 60 days respectively). The expression of XRCC1 mRNA in brain was detected by RT-PCR tech⁃nique after separation of cerebral cortex, cerebellum, and hippocampus. At the same time, lead content in brain tissue and catalase (CAT), glutathione (GSH) and hydrogen peroxide (H2O2)were also detected. Results Compared with control group, the expression of XRCC1 mRNA, content of lead and H2O2levels were significantly higher in cerebral cortex, cerebellum and hippocampus of exposure groups (P < 0.05), whereas the contents of CAT and GSH were significantly lower (P < 0.05). There was a positive correlation between lead level and the expression of XRCC1 mRNA in cerebral cortex, cerebellum and hippo⁃campus of exposure groups (r=0.608, 0.438 and 0.470,P<0.01). There was a negative correlation between the lead level and CAT and GSH (r=-0.343, -0.465、-0.423, -0.383, -0.489 and -0.366,P<0.05). A positive relationship was found between the lead level and H2O2(r=0.455, 0.517 and 0.342,P<0.05). ConclusionLead exposure can affect the expression of mRNA gene in XRCC1 through inducing cell oxidative stress.
Key words:lead; XRCC1; oxidative stress; catalase; glutathione; hydrogen peroxide; brain tissue
中图分类号:R994.4
文献标志码:A
DOI:10.11958/20150151
基金项目:国家自然科学基金资助项目(81160342);江西省自然科学基金资助项目(20122BAB205047);江西省教育厅科技项目(GJJ11312)
作者简介:李炜娟(1988),女,硕士研究生,主要从事重金属中毒机制与防治研究



