崔蕾,郭飞,闫晔,潘明霞,董羊羊,薛凤霞
血清FSH、LH、PRL与浆液性卵巢癌临床病理特征及预后的关系
崔蕾,郭飞,闫晔,潘明霞,董羊羊,薛凤霞△
目的探讨血清卵泡刺激素(FSH)、黄体生成素(LH)、催乳素(PRL)与浆液性卵巢癌临床病理特征及预后的关系。方法选择2000年1月—2015年12月在天津医科大学总医院妇产科住院治疗的浆液性卵巢癌患者73例。采用Mann-Whitney U检验分析患者血清FSH、LH、PRL与临床病理特征的关系,采用Kaplan-Meier(K-M)分析不同临床病理特征患者生存率差异,多因素COX比例风险回归模型分析浆液性卵巢癌患者预后的影响因素。结果年龄>50岁组血清FSH、LH水平显着高于年龄≤50岁组,绝经组血清FSH、LH水平显着高于未绝经组(均P<0.05),其他不同临床病理特征分组患者间血清FSH、LH水平差异无统计学意义;不同临床病理特征分组患者间血清PRL水平差异均无统计学意义。单因素分析显示,血清FSH水平高(>40.13 IU/L)、血清PRL水平高(>14.96 μg/L)、FIGO分期(Ⅲ+Ⅳ)的患者预后差(均P<0.05);血清LH水平与患者预后无关(P>0.05)。COX多因素回归分析显示,血清 PRL>14.96 μg/L 是影响浆液性卵巢癌预后的危险因素[HR(95%CI)为 3.530(1.180~10.557),P=0.024]。结论绝经后妇女血清中FSH、LH水平较绝经前显着升高,PRL与浆液性卵巢癌患者预后有关。
卵巢肿瘤;卵泡刺激素;促黄体激素;催乳素;预后;浆液性卵巢癌
5年生存率在30%~40%[2]。流行病学研究发现浆液性卵巢癌好发于绝经后妇女,口服避孕药、妊娠(尤其是多胎妊娠)及哺乳期延长可以降低促性腺激素水平,从而降低卵巢癌的发生风险;而初潮早、绝经迟、多囊卵巢综合征(PCOS)妇女体内促性腺激素水平高,患卵巢癌风险增加[3],提示内分泌因素对于卵巢癌的发生具有一定的作用[4]。体外实验证实卵泡刺激素(FSH)、黄体生成素(LH)、催乳素(PRL)能够促进卵巢癌细胞的增殖、迁移及侵袭[5],抑制卵巢癌细胞凋亡[6],诱导卵巢上皮发生上皮间质转化(EMT)[7],提示 FSH、LH、PRL 有可能参与卵巢癌的进展。目前国内外对于FSH、LH、PRL与卵巢癌关系的研究主要侧重于机制方面。本研究通过分析
73例浆液性卵巢癌患者术前血清FSH、LH、PRL水平与浆液性卵巢癌患者临床病理特征及预后的关系,旨在为评估浆液性卵巢癌患者预后提供依据。
1 资料与方法
1.1 一般资料 选择2000年1月—2015年12月在天津医科大学总医院妇产科住院治疗的浆液性卵巢癌患者,均行手术治疗,术后病理证实为浆液性卵巢癌,且术前未行放疗、化疗或激素治疗,术前均行性激素六项检查的患者73例纳入本研究。患者年龄 38~82岁,平均(58.78±9.44)岁。
1.2 方法 血清FSH、LH、PRL、患者的年龄、FIGO分期、病理分级、是否绝经、腹水细胞学检查结果、腹水量、肿瘤直径等临床及病理资料均从病历资料中获取。按卵巢上皮性癌2013年国际妇产科联盟(FIGO)新分期标准重新分期,请病理医生重新复核病理分级。肿瘤直径、腹水量、血清FSH、LH、PRL 的中位数分别为 10 cm、300 mL、40.13 IU/L、24.9 IU/L、14.96 μg/L,以中位数作为临界值进行分组。对73例患者进行随访,随访起始时间为患者确诊为浆液性卵巢癌的时间,通过电话或门诊等方式对患者进行随访,截止时间为2016年12月31日。总生存时间(OS)定义为从确诊到死于浆液性卵巢癌的时间,失访、随访截止时仍健在以及患者死于其他疾病定义为删失。本研究所使用资料均取得患者或家属的知情同意。
1.3 统计学方法 采用SPSS 16.0进行统计学处理。计量资料服从正态分布者以±s表示;不服从正态分布者以M(P25,P75)表示,组间比较采用Mann-Whitney U检验。单因素生存分析应用Kaplan-Meier法,组间比较行Log-rank检验。多因素生存分析应用COX回归分析。P<0.05为差异有统计学意义。
2 结果
2.1 血清FSH、LH、PRL水平与患者临床病理特征之间的关系 年龄>50岁组血清FSH、LH水平高于年龄≤50岁组,绝经组血清FSH、LH水平高于未绝经组(均P<0.01),其余不同临床病理特征分组患者间血清FSH、LH水平差异无统计学意义。不同临床病理特征分组患者间血清PRL水平差异均无统计学意义,见表1。

Tab.1 Relationship between serum FSH,LH,PRL and different clinicopathological characteristics in patients with serous ovarian cancer表1 血清FSH、LH、PRL与浆液性卵巢癌临床病理特征之间的关系 M(P25,P75)
2.2 随访结果 随访时间2.1~175.7个月,中位随访时间29.3个月;死亡14例,病死率19.18%;失访8例,失访率10.96%。
2.3 生存分析
2.3.1 单因素分析 临床分期晚[(Ⅲ+Ⅳ)期]、血清FSH>40.13 IU/L、血清 PRL>14.96 μg/L 的患者预后较差(P<0.05);其余不同临床病理特征患者间生存率差异无统计学意义,见表2。

Tab.2 Univariate analysis of serum FSH,LH,PRL and prognosis in patients with serous ovarian cancer表2 血清FSH、LH、PRL与浆液性卵巢癌患者预后的单因素分析
2.3.2 多因素分析 以血清FSH(>40.13 IU/L=1,≤40.13 IU/L=0)、血清 PRL(>14.96 μg/L=1,≤14.96 μg/L=0)为自变量,以生存结局(生存或失访=0,死亡=1)和生存时间为因变量,进行多因素分析。结果显示血清PRL>14.96 μg/L是影响浆液性卵巢癌患者死亡的危险因素(P=0.024),见表3,生存曲线见图1。
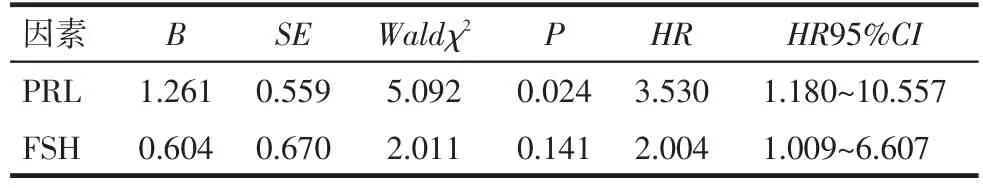
Tab.3 Analysis of prognostic factors in patients with serous ovarian cancer表3 浆液性卵巢癌患者预后的影响因素分析

Fig.1 Survival curves of serum PRL and clinical prognosis of patients with serous ovarian cancer(COX regression analysis)图1 血清PRL与浆液性卵巢癌患者预后的生存曲线
3 讨论
流行病学研究显示卵巢癌好发于绝经后妇女,原因可能为绝经后妇女雌激素水平降低,诱导下丘脑释放促性腺激素释放激素(GnRH),进而刺激垂体释放FSH和LH所致,其中释放FSH较LH更多。本研究73例浆液性卵巢癌患者中绝经者56例,未绝经者17例,绝经后血清FSH、LH水平较绝经前明显升高,符合卵巢癌流行病学特征。既往研究发现PCOS患者、围绝经期妇女及因无排卵而接受促性腺激素治疗的妇女血FSH、LH水平升高而致患卵巢癌风险增加[8-9],提示FSH、LH可能参与卵巢癌的发生[10]。体外实验证实FSH、LH可诱导卵巢上皮发生EMT[11],促进 EOC 细胞增殖、迁移、侵袭[4-5],抑制EOC细胞凋亡[6,12],提示FSH、LH可能参与卵巢癌的发展。Chudecka-Glaz等[13]发现EOC患者血清及囊性肿瘤内的液体中的FSH、LH水平均明显高于良性对照组。Chen等[14]发现复发组卵巢癌患者血清FSH水平显着高于初发组,血清中FSH、LH水平与腹水中FSH、LH水平呈正相关,但腹水中FSH、LH水平并未超过血清中水平,提示腹水中FSH、LH可能来源于血清超滤液而并非腹膜产生;经多因素分析显示腹水中高水平FSH是影响EOC患者预后的独立危险因素,这提示血清FSH、LH水平可能与EOC患者腹水量有关。本研究发现不同腹水量的浆液性卵巢癌患者血清FSH、LH水平差异无统计学意义,血清FSH、LH水平与腹水中FSH、LH水平及腹水量之间的关系仍需进一步探讨。本研究还发现血清高水平FSH与浆液性卵巢癌患者预后差有关,提示血清高水平FSH可能通过改变浆液性卵巢癌患者体内的内分泌环境,参与了卵巢癌的发生[9]。血清FSH有望成为评估浆液性卵巢癌预后的有价值的新指标。
近年来有文献报道PRL及其受体在多种肿瘤细胞中均有表达[15-16],PRL可促进肿瘤细胞的增殖、侵袭、迁移[17-18]。Wang 等[19]研究发现:乳腺癌患者血清PRL水平较正常妇女明显升高;血清PRL有望成为评估乳腺癌预后的指标;PRL抑制剂有利于提高血清高PRL乳腺癌患者的化疗敏感性。在卵巢癌组织[20]、增生的子宫内膜及子宫平滑肌细胞中均可以测出高水平的PRL,提示PRL与女性生殖系统肿瘤关系密切[21]。目前关于PRL与卵巢癌关系的研究较少。既往研究发现分娩及口服避孕药等可降低PRL水平[22],从而降低卵巢癌发病率,高水平PRL增加卵巢癌发病风险,PRL可以作为卵巢癌诊断的血清标志物之一[23]。体外实验证实PRL可以抑制BRCA1基因的抑癌作用[24],促进卵巢癌细胞的增殖、迁移、侵袭[23],抑制卵巢癌细胞凋亡[24],提示PRL参与了卵巢癌的发生发展。本研究发现,血清PRL水平高者预后差,血清PRL是影响浆液性卵巢癌预后的危险因素。血清高水平PRL可能通过改变浆液性卵巢癌患者体内的内分泌环境[22],促进卵巢上皮细胞增殖、迁移、侵袭[23],抑制抑癌基因发挥作用及铂类化疗药物诱导的细胞凋亡[20,24],参与浆液性卵巢癌的发生发展。PRL有望成为评估浆液性卵巢癌预后的指标之一。本研究还发现,不同FIGO分期及组织学分级的浆液性卵巢癌患者血清FSH、PRL水平无明显差异,这与既往研究类似[14,25]。FSH、PRL及其受体在卵巢和输卵管中表达,作为内分泌和旁分泌信号分子参与卵巢癌的发生[25]。原发卵巢癌组织即卵巢和输卵管中的PRL水平,可能在卵巢癌的发生发展中发挥更关键的作用。而本研究中血清FSH、PRL水平可能未能准确反映原发卵巢癌组织中PRL的水平。目前国内外关于血清PRL水平与浆液性卵巢癌患者预后相关性的报道较少,因此本文结论尚需多中心大样本研究进一步证实。
[1]Siegel RL,Miller KD,Jemal A.Cancer Statistics,2017[J].CA Cancer J Clin,2017,67(1):7-30.doi:10.3322/caac.21387.
[2]Allemani C,Weir HK,Carreira H,et al.Global surveillance of cancer survival 1995-2009:analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries(CONCORD-2)[J].Lancet,2015,385(9972):977-1010.doi:10.1016/S0140-6736(14)62038-9.
[3]Sung HK,Ma SH,Choi JY,et al.The Effect of breast feeding duration and parity on the risk of epithelial ovarian cancer:a systematic review and meta-analysis[J].J Prev Med Public Health,2016,49(6):349-366.doi:10.3961/jpmph.16.066.
[4]Bachelot A,Carre N,Mialon O,et al.The permissive role of prolactin as a regulator of luteinizing hormone action in the female mouse ovary and extragonadal tumorigenesis[J].Am J Physiol Endocrinol Metab,2013,305(7):E845-E852.doi:10.1152/ajpendo.00243.2013.
[5]Yang X,Wang J,Li WP,et al.Desmocollin 3 mediates follicle stimulating hormone-induced ovarian epithelialcancercell proliferation by activating the EGFR/Akt signaling pathway[J].Int J Clin Exp Pathol,2015,8(6):6716-6723.
[6]Xia L,Wen H,Han X,et al.Luteinizing hormone inhibits cisplatininduced apoptosis in human epithelial ovarian cancer cells[J].Oncol Lett,2016,11(3):1943-1947.doi:10.3892/ol.2016.4122.
[7]Miow QH,Tan TZ,Ye J,et al.Epithelial-mesenchymal status renders differential responses to cisplatin in ovarian cancer[J].Oncogene,2015,34(15):1899-1907.doi:10.1038/onc.2014.136.
[8]Wang Y,Cai KQ,Smith ER,et al.Follicle depletion provides a permissive environment for ovarian carcinogenesis[J].Mol Cell Biol,2016,36(18):2418-2430.doi:10.1128/MCB.00202-16.
[9]Lauretta R,Lanzolla G,Vici P,et al.Insulin-sensitizers,polycystic ovary syndrome and gynaecologicalcancerrisk [J].IntJ Endocrinol,2016,2016:8671762.doi:10.1155/2016/8671762.
[10]Lee AW,Tyrer JP,Doherty JA,et al.Evaluating the ovarian cancer gonadotropin hypothesis:a candidate gene study[J].Gynecol Oncol,2015,136(3):542-548.doi:10.1016/j.ygyno.2014.12.017.
[11]Liu L,Zhang J,Fang C,et al.OCT4 mediates FSH-induced epithelial-mesenchymal transition and invasion through the ERK1/2 signaling pathway in epithelial ovarian cancer[J].Biochem Biophys Res Commun,2015,461(3):525-532.doi:10.1016/j.bbrc.2015.04.061.
[12]Rossi V,Lispi M,Longobardi S,et al.LH prevents cisplatininduced apoptosis in oocytes and preserves female fertility in mouse[J].Cell Death Differ,2017,24(1):72-82.doi:10.1038/cdd.2016.97.
[13]Chudecka-Glaz A,Rzepka-Gorska I,Kosmowska B.Gonadotropin(LH,FSH)levels in serum and cyst fluid in epithelial tumors of the ovary[J].Arch Gynecol Obstet,2004,270(3):151-156.doi:10.1007/s00404-003-0519-4.
[14]Chen FC,Oskay-Ozcelik G,Buhling KJ,et al.Prognostic value of serum and ascites levels of estradiol,FSH,LH and prolactin in ovarian cancer[J].Anticancer Res,2009,29(5):1575-1578.
[15]O’Sullivan CC,Bates SE.Targeting prolactin receptor(PRLR)signaling in PRLR-positive breast and prostate cancer[J].Oncologist,2016,21(5):523-526.
[16]Kong X,Wu W,Yuan Y,et al.Human growth hormone and human prolactin function as autocrine/paracrine promoters of progression of hepatocellular carcinoma[J].Oncotarget,2016,7(20):29465-29479.doi:10.18632/oncotarget.8781.
[17]Hammer A,Diakonova M.Prolactin-induced PAK1 tyrosyl phosphorylation promotes FAK dephosphorylation,breast cancer cell motility,invasion and metastasis[J].BMC Cell Biol,2016,17(1):31.doi:10.1186/s12860-016-0109-5.
[18]Abdelbaset-Ismail A,Pedziwiatr D,Schneider G,et al.Pituitary sex hormones enhance the prometastatic potential of human lung cancer cells by downregulating the intracellular expression of heme oxygenase-1[J].Int J Oncol,2017,50(1):317-328.doi:10.3892/ijo.2016.3787.
[19]Wang M,Wu X,Chai F,et al.Plasma prolactin and breast cancer risk:a meta-analysis[J].Sci Rep,2016,6:25998.doi:10.1038/srep25998.
[20]Wen Y,Zand B,Ozpolat B,et al.Antagonism of tumoral prolactin receptor promotes autophagy-related cell death[J].Cell Rep,2014,7(2):488-500.doi:10.1016/j.celrep.2014.03.009.
[21]Schock H,Zeleniuch-Jacquotte A,Lundin E,et al.Hormone concentrations throughout uncomplicated pregnancies: a longitudinal study[J].BMC Pregnancy Childbirth,2016,16(1):146.doi:10.1186/s12884-016-0937-5.
[22]Clendenen TV,Arslan AA,Lokshin AE,et al.Circulating prolactin levels and risk of epithelial ovarian cancer[J].Cancer Causes Control,2013,24(4):741-748.doi:10.1007/s10552-013-0156-6.
[23]Tan D,Chen KE,Khoo T,et al.Prolactin increases survival and migration of ovarian cancer cells:importance of prolactin receptor type and therapeutic potential of S179D and G129R receptor antagonists[J].Cancer Lett,2011,310(1):101-108.doi:10.1016/j.canlet.2011.06.014.
[24]ChenKH,WalkerAM.Prolactininhibitsamajortumorsuppressive function of wild type BRCA1[J].Cancer Lett,2016,375(2):293-302.doi:10.1016/j.canlet.2016.03.007.
[25]刘贵鹏,张小围,王爽,等.催乳素与卵巢癌的相关性分析[J].广东医学,2012,33(21):3291-3293.Liu GP,Zhang XW,Wang S,et al.Correlation between prolactin and ovarian cancer[J].Guangdong Medical Journal,2012,33(21):3291-3293.doi:10.3969/j.issn.1001-9448.2012.21.040.
(2017-02-14收稿 2017-04-26修回)
(本文编辑 李国琪)
The relationship of serum levels of FSH,LH and PRL and clinicopathological features and prognosis in patients with serous ovarian cancer
CUI Lei,GUO Fei,YAN Ye,PAN Ming-xia,DONG Yang-yang,XUE Feng-xia△
Department of Obstetrics and Gynecology,Tianjin Medical University General Hospital,Tianjin 300052,China△
E-mail:fengxiaxue1962@163.com
ObjectiveTo investigate the relationship between serum follicle stimulating hormone(FSH),luteinizing hormone(LH),prolactin(PRL)and clinicopathological features and prognosis of serous ovarian cancer retrospectively.MethodsA total of 73 patients with serous ovarian cancer treated in the Department of Obstetrics and Gynecology of Tianjin Medical University General Hospital from January 2000 to December 2015 were included in this study.The relationship between serum FSH,LH,PRL and clinicopathological features was analyzed by Mann-Whitney U method.Kaplan-Meier(K-M)method was used to analyze survival rates of patients with different clinical features.Multivariate Cox proportional hazards regression analysis was used to analyze prognostic factors of serous ovarian cancer patients.ResultsThe mean concentrations of serum FSH and LH were significantly higher in the>50 year-old group than those in the<50 year-old group(P<0.05).The mean concentrations of FSH and LH were significantly higher in menopause group than those in non-menopause group(P<0.05).There were no significant differences in serum levels of FSH and LH in patients with other different clinicopathological features(P>0.05).There was no significant correlation between serum PRL concentration and clinicopathological features(P>0.05).Analysis results showed that poor prognosis of patients was related with high serum levels of FSH(>40.13 IU/L),PRL(>14.96 μg/L)and FIGO stage(Ⅲ+Ⅳ)(P<0.05).There was no significant correlation between serum LH concentration and prognosis(P>0.05).COX regression analysis showed that the serum PRL>14.96 μg/L was risk factor for prognosis of serous ovarian cancer[HR(95%CI):3.530(1.180-10.557),P=0.024].ConclusionThe serum levels of FSH and LH are significantly increased in postmenopausal women than those in menopause women.The serum level of PRL is correlated with the prognosis of serous ovarian cancer.
ovarian neoplasms;follicle stimulating hormone;luteinizing hormone;prolactin;prognosis;serous ovarian cancer卵巢癌是目前死亡率最高的妇科恶性肿瘤,美国2016年新增卵巢癌22 280例,死亡4 240例,其死亡率明显高于宫颈癌和子宫内膜癌[1]。卵巢癌最常见的病理类型为上皮性卵巢癌(epithelial ovarian cancer,EOC),其中以浆液性卵巢癌最常见。据统计,70%的浆液性卵巢癌患者就诊时已处于晚期,其
R737.31
:A
10.11958/20170184
国家自然科学基金青年项目(81602292)
天津医科大学总医院妇产科(邮编300052)
崔蕾(1991),女,硕士在读,主要从事妇科肿瘤方面研究
△通讯作者 E-mail:fengxiaxue1962@163.com



