高光仁 冯连荣 付金国 郭润 牛和平 李凤鹏 张倩玉 张军


摘要:目的 探究合并胸腔积液的急性心肌梗死(AMI)患者的心肌损伤特点及对远期预后的影响。方法 前瞻性连续入选发病15 d内住院且行心脏磁共振成像及心脏彩超检查的AMI患者。按照心脏彩超有无胸腔积液分为有胸腔积液组和无胸腔积液组。比较2组患者基线资料,心脏磁共振心肌损伤指标及心脏彩超特征。通过门诊复诊、电话等方式随访,记录其主要不良心脑血管事件,包括全因死亡、再发心肌梗死、再次冠状动脉血运重建、因心力衰竭再入院及卒中的复合终点。Cox回归分析患者发生全因死亡的影响因素。结果 211例中有胸腔积液31例(14.7%),无胸腔积液180例(85.3%)。与无胸腔积液组比较,有胸腔积液组心脏彩超左心室舒张末期直径更大,左心室射血分数更低(P<0.05);心脏磁共振成像示2组患者梗死面积、左心室舒张末期容积、左心室收缩末期容积、左心室射血分数、微循环阻塞及心肌内出血发生率比较差异无统计学意义(均P>0.05)。随访显示43例(20.4%)发生主要不良心脑血管事件,2组比较差异无统计学意义(χ2=3.160,P=0.075);其中6例(2.8%)发生全因死亡,有胸腔积液组全因死亡发生率高于无胸腔积液组(9.7% vs. 1.7%,P<0.05),2组的其他不良事件发生率差异均无统计学意义(均P>0.05);多因素Cox回归分析示高龄及发生胸腔积液是随访发生全因死亡的独立危险因素。结论 AMI合并胸腔积液患者的心肌损伤更重,全因死亡率更高。
关键词:心肌梗死;胸腔积液;预后;磁共振成像;超声心动描记术,多普勒,彩色;心肌损伤
中图分类号:R542.22文献标志码:ADOI:10.11958/20230377
Characteristics of myocardial injury in patients with acute myocardial infarction complicated with pleural effusion and its influence on long-term prognosis
Abstract: Objective To explore the characteristics of myocardial injury in patients with acute myocardial infarction (AMI) complicated by pleural effusion and its effect on long-term prognosis. Methods It was a prospective single-center study. Patients with AMI who were admitted to hospital within 15 days from symptom onset and performed echocardiography and cardiac magnetic resonance imaging (CMR) during hospitalization were consecutively enrolled and assigned to the with-pleural effusion group and the without-pleural effusion group according to the echocardiography result. Baseline data, cardiac magnetic resonance myocardial injury index and echocardiography characteristics were compared between the two groups. The occurrence of major adverse cardiovascular and cerebrovascular events (MACCE) was recorded through outpatient follow-up and telephone follow-up, including all-cause death, re-infarction, revascularization, rehospitalization for congestive heart failure and stroke. Cox regression analysis was performed to analyze influencing factors of all-cause death. Results Among 211 patients, 31 (14.7%) patients had pleural effusion and 180 (85.3%) had no pleural effusion. Compared with the group without pleural effusion, the left ventricular end-diastolic diameter was larger, and left ventricular ejection fraction assessed by echocardiography was lower in the group with pleural effusion (P<0.05). There were no significant differences in infarct size, left ventricular end-diastolic volume, left ventricular end-systolic volume, left ventricular ejection fraction and the presence of microvascular obstruction and intramyocardial hemorrhage between the two groups in CMR (all P>0.05). At a median follow-up of 31 months, MACCE occurred in 43 (20.4%) patients, and there was no significant difference between the two groups (χ2=3.160,P=0.075). Six cases (2.8%) had all-cause death. The incidence of all-cause death was higher in the group with pleural effusion than that in the group without pleural effusion (9.7% vs. 1.7%, P < 0.05). There was no significant difference in the incidence of other adverse events between the two groups (P>0.05). Multivariate Cox regression analysis showed that advanced age and presence of pleural effusion were independent risk factors of all-cause death during follow-up. Conclusion Patients with AMI combined with pleural effusion have more severe myocardial injury and higher all-cause mortality.
Key words: myocardial infarction; pleural effusion; prognosis; magnetic resonance imaging; echocardiography, Doppler, color; myocardial injury
急性心肌梗死(AMI)是目前危害我国居民健康的主要疾病之一,尽管近年通过急诊经皮冠状动脉介入治疗(PCI)及时地开通急性闭塞的罪犯血管挽救了大量患者的生命,但其病死率在我国仍然呈整体上升趋势[1]。目前已知AMI预后的预测因子包括吸烟、动态心电图监测的平均心率、入院血清D-二聚体等[1]。AMI急性期发生胸腔积液可能是心脏损伤后综合征(post-cardiac injury syndrome,PCIS)的症状之一[2-4],但其对AMI预后的影响目前报道较少。本研究旨在探索AMI合并胸腔积液患者的临床特征、心脏磁共振评价的心肌损伤严重程度,并探讨胸腔积液对AMI远期预后的影响。
1 对象与方法
1.1 研究对象 作为前瞻性研究连续纳入2018年10月—2021年10月沧州市中心医院心内科住院患者。入选标准:(1)参考2012年全球心肌梗死定义第3版诊断为AMI。(2)18~80岁。(3)住院期间完成心脏磁共振成像及心脏彩超检查。排除标准:(1)发病至入院超过15 d。(2)既往有陈旧性心肌梗死病史。
1.2 治疗方案 患者入院常规完成实验室检查;患者住院期间均行冠状动脉造影,并依据目前指南行介入治疗。患者发病3~15 d内行心脏磁共振及心脏彩超检测。所有患者住院期间每日给予阿司匹林100 mg,替格瑞洛180 mg或氯吡格雷75 mg。其余药物如他汀类、β受体阻滞剂、血管紧张素转换酶抑制剂(ACEI)、血管紧张素受体拮抗剂(ARB)等按照指南推荐服用。本研究经本院伦理委员会批准(2020-286-01),患者或家属均签署知情同意书。
1.3 资料收集 收集患者性别、年龄、入院收缩压、梗死类型及位置、既往史、介入情况、实验室检查及住院用药等临床资料,梗死面积(infarct size,IS)、微循环阻塞(microvascular obstruction,MVO)、心肌内出血(intramyocardial hemorrhage,IMH)、左心室射血分数(left ventricular ejection fraction,LVEF)、左心室舒张末期容积(left ventricular end-diastolic volume,LVEDV)及左心室收缩末期容积(left ventricular end-systolic volume,LVESV)等心肌损伤和心功能指标以及LVEF、左心室舒张末期直径(left ventricular end-diastolic diameter, LVEDD)、左心房内径(left atrial diameter,LAD)等心脏超声指标。IS定义为延迟强化区域体积占左心室总体积的百分比(%LV)[5]。心脏磁共振检查、测量方法及指标定义参考既往研究[6]。磁共振影像数据由2名具有5年以上工作经验的磁共振专业医生分析,对分组不知情。按心脏彩超有无胸腔积液将患者分为有胸腔积液组和无胸腔积液组。
1.4 随访 采用门诊随访或对患者本人或家属电话随访,优先选择对患者本人随访,随访截至2022年11月7日。研究终点为发生主要不良心脑血管事件(major adverse cardiovascular and cerebrovascular events,MACCE),包括全因死亡、再发心肌梗死、再次冠状动脉血运重建、因心力衰竭再入院及卒中的复合终点。发生MACCE的患者以事件发生时间为最后随访时间,其他患者记录实际随访时间。
1.5 统计学方法 采用SPSS 22.0软件进行数据处理。正态分布的计量资料以均数±标准差([[x] ±s
])表示,组间比较采用t检验。计数资料以例(%)表示,组间比较采用χ2检验或Fisher精确概率法。非正态分布的计量资料以M(P25,P75)表示,组间比较采用Mann-Whitney U检验。采用Cox比例风险回归模型(向后Wald法)分析胸腔积液对预后的影响,Kaplan-Meier法绘制生存曲线,Log-rank检验比较生存率的差异。P<0.05为差异有统计学意义。
2 结果
2.1 2组基线资料的比较 共纳入AMI患者211例,其中有胸腔积液组31例(14.7%),无胸腔积液组180例(85.3%)。2组在性别、年龄、入院收缩压、梗死类型及位置、既往史、介入情况、实验室检查及住院用药方面差异无统计学意义,见表1。
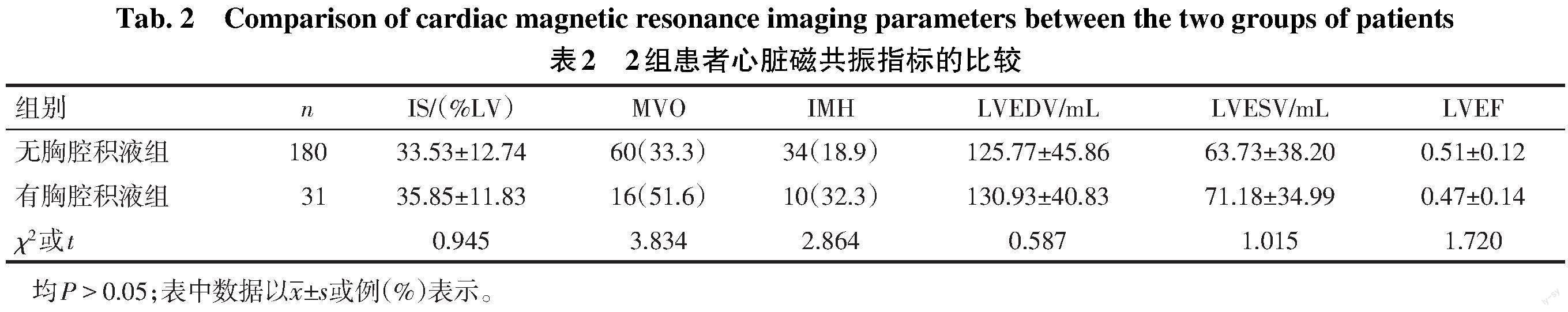
2.2 2组心脏磁共振及心脏彩超指标的比较 2组患者磁共振指标中MVO和IMH发生率及IS、LVEDV、LVESV、LVEF比较差异均无统计学意义(均P>0.05),见表2。有胸腔积液组心脏彩超LVEDD更大,LVEF更低(P<0.05),但2组LAD差异无统计学意义(P>0.05),见表3。
2.3 2组患者MACCE发生情况比较 中位随访期为31(23,39)个月。6例(2.8%)发生全因死亡,其中有胸腔积液组3例(9.7%),无胸腔积液组3例(1.7%);10例(4.7%)发生再梗死;8例(3.8%)因心衰再入院;21例(10.0%)发生再血管化;6例(2.8%)发生卒中;合计43例(20.4%)发生MACCE。有胸腔积液组全因死亡发生率高于无胸腔积液组(P<0.05),2组其余指标差异均无统计学意义(均P>0.05),见图1。
2.4 2组患者的生存情况比较 Kaplan-Meier生存曲线显示有胸腔积液组的累积生存率低于无胸腔积液组(Log-rank χ2=5.446,P=0.020),见图2。
2.5 全因死亡影响因素的Cox回归分析 以是否全因死亡作为因变量(是=1,否=0),将基线资料(表1)与心脏磁共振及彩超指标(表2、3)中比较P<0.1的各指标及年龄与发生胸腔积液作为自变量,包括年龄、LVEDD、心脏彩超评价LVEF、MVO(有=1,无=0)、IMH(有=1,无=0)及胸腔积液(发生=1,未发生=0),进行单因素Cox回归分析,结果显示高龄及发生胸腔积液是发生全因死亡的危险因素(P<0.05),见表4。将年龄、胸腔积液纳入多因素Cox分析,结果显示高龄及发生胸腔积液是患者发生全因死亡的独立危险因素(P<0.05),见表5。
3 讨论
胸腔积液是临床常见的胸膜病变,也是多种疾病的伴随表现,包括渗出液和漏出液2种类型,不同类型疾病患者产生的胸腔积液有其各自的特点[7]。PCIS的主要临床表现即包含胸腔积液,但PCIS的发病机制尚不明确,一种假说认为心脏损伤后心脏抗原释放入血液循环,高循环水平的抗原可结合自身抗体形成免疫复合物沉积于心包、胸膜、肺和关节,进一步诱发心包积液、胸腔积液、肺实质性改变及关节腔积液的发生[4]。本研究探讨了合并胸腔积液的AMI患者心肌受损特点,尝试从心肌损伤方面探讨胸腔积液的形成机制及对预后的影响。
本研究2组的磁共振成像指标,如IS、MVO、IMH、LVEF差异虽然无统计学意义,但在有胸腔积液组较无胸腔积液组均趋向更严重。AMI患者心肌梗死面积与心功能受损严重程度有关[8],且STEMI患者出现MVO与梗死面积增大、发生左心室重构及射血分数降低有关[9-10]。MVO是指心脏磁共振延迟钆增强区域中的暗区,由冠状动脉再灌注诱发,为“无复流”现象的影像表现,其延长了心肌缺血时间[11]。晚期MVO被认为是随访出现心脏舒张末期容积及收缩末期容积增大、LVEF下降的最强预测因素;同时,发生晚期MVO也是左心室重构的替代指标[12]。MVO影响了梗死心肌的修复过程,导致心肌可能出现3种模式:正常修复、心肌扩张伴随功能性适应及心肌扩张未伴随功能性适应[13]。结合本研究,笔者推测有胸腔积液组MVO增多介导了心肌扩张,LVEDD增加,但未伴随功能性适应,进而左心室收缩功能下降,LVEF降低,最终出现胸腔积液。
既往研究认为,合并胸腔积液是急性肺水肿患者1年内发生死亡的独立危险因素[14]。本研究经随访发现,合并胸腔积液是AMI发生全因死亡的危险因素。急性肺水肿或AMI发生时均出现心功能下降及静脉压增高,肺循环淤血可能为发生胸腔积液的主要原因。本研究发现,虽然有胸腔积液组心脏彩超评价的LVEF小于无胸腔积液组,但仍大于0.50,考虑患者处于射血分数保留的心力衰竭中的临床心力衰竭阶段[15-16]。多因素Cox回归分析未提示LVEF为AMI患者发生远期全因死亡的独立危险因素,这提示胸腔积液较LVEF更敏感,在LVEF尚在正常阶段就能够对AMI患者进行早期危险分层。
综上所述,AMI合并胸腔积液患者的心肌损伤更重,全因死亡率更高。本研究中磁共振成像各指标包括IS、MVO、IMH、LVEF等在有胸腔积液组较无胸腔积液组均趋向更严重,但差异无统计学意义,这可能受样本量较小影响,因此需要今后更大样本研究证实2组心肌受损差异。本研究未对胸腔积液做诊断性穿刺确定病因,且由于为小样本队列研究,未按照积液量大小分组进一步做敏感性分析,因此胸腔积液对AMI心肌损伤的影响及预后预测价值仍需大样本、多中心前瞻性研究进一步证实。
参考文献
[1] 中国心血管健康与疾病报告编写组. 中国心血管健康与疾病报告2021概要[J]. 中国循环杂志,2022,37(6):553-578. The Writing Committee of the Report on Cardiovascular Health and Diseases in China. Report on cardiovascular health and diseases in China 2021:An updated summary[J]. Chinese Circulation Journal,2022,37(6):553-578. doi:10.3969/j.issn.1000-3614.2022.06.001.
[2] JARYAL A,VIKRANT S,SARKAR M,et al. A case report of pleuro-pericardial effusion in a patient on hemodialysis and a cardiac pacemaker[J]. Semin Dial,2021,34(4):323-325. doi:10.1111/sdi.12996.
[3] NISHIMURA M,GODA N,HATAZAWA K,et al. Delayed diagnosis of postcardiac injury syndrome[J]. BMJ Case Rep,2019,12(2):e228877. doi:10.1136/bcr-2018-228877.
[4] 殷鑫,张雨,邵玥明,等. 心脏损伤后综合征再发与治疗体会[J]. 天津医药,2020,48(6):555-557. YIN X,ZHANG Y,SHAO Y M,et al. The clinical experience of recurrence and treatment of post-cardiac injury syndrome[J]. Tianjin Med J,2020,48(6):555-557. doi:10.11958/20192963.
[5] 王佳丽,孔莹,孙小伶,等. 磁共振对急性ST段抬高型心肌梗死介入术后左心功能改善的预测价值[J]. 磁共振成像,2022,13(2):87-90. WANG J L,KONG Y,SUN X L,et al. Predictive value of MRI for cardiac function improvement after intervention in acute myocardial infarction patients[J]. Chin J Magn Reson Imaging,2022,13(2):87-90. doi:10.12015/issn.1674-8034.2022.02.018.
[6] GAO G,FENG L,FU J,et al. Prognostic value of the SYNTAX score on myocardial injury and salvage in STEMI patients after primary percutaneous coronary intervention:A single-center retrospective observational study[J]. BMC Cardiovasc Disord,2021,21(1):591. doi:10.1186/s12872-021-02395-7.
[7] 张秀强,杨涛. 胸腔积液的特点及其鉴别诊断的研究进展[J]. 国际生物医学工程杂志,2022,45(6):563-567. ZHANG X Q,YANG T. Research progress in the characteristics and differential diagnosis of tuberculous pleural effusion and malignant pleural effusion[J]. International Journal of Biomedical Engineering,2022,45(6):563-567. doi:10.3760/cma.j.cn121382-20220715-00617.
[8] 王建林,王琰华,史磊. 急性心肌梗死患者LGE-CMR评价结果及其与血清心肌标志物的关系[J]. 天津医药,2022,50(4):393-398. WANG J L,WANG Y H,SHI L. Relationship between evaluation results of LGE-CMR and serum myocardial markers in patients with acute myocardial infarction[J]. Tianjin Med J,2022,50(4):393-398. doi:10.11958/20211980.
[9] DAVIDSON S J,RONCALLI J,SURDER D,et al. Microvascular obstruction identifies a subgroup of patients who benefit from stem cell therapy following ST-elevation myocardial infarction[J]. Am Heart J,2023,259:79-86. doi:10.1016/j.ahj.2023.02.004.
[10] MASCI P G,PAVON A G,PONTONE G,et al. Early or deferred cardiovascular magnetic resonance after ST-segment-elevation myocardial infarction for effective risk stratification[J]. Eur Heart J Cardiovasc Imaging,2020,21(6):632-639. doi:10.1093/ehjci/jez179.
[11] CALVIERI C,RIVA A,STURLA F,et al. Left ventricular adverse remodeling in ischemic heart disease: emerging cardiac magnetic resonance imaging biomarkers[J]. J Clin Med,2023,12(1):334. doi:10.3390/jcm12010334.
[12] HAMIRANI Y S,WONG A,KRAMER C M,et al. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta-analysis[J]. JACC Cardiovasc Imaging,2014,7(9):940-952. doi:10.1016/j.jcmg.2014.06.012.
[13] ?RN S,MANHENKE C,GREVE O J,et al. Microvascular obstruction is a major determinant of infarct healing and subsequent left ventricular remodelling following primary percutaneous coronary intervention[J]. Eur Heart J,2009,30(16):1978-1985. doi:10.1093/eurheartj/ehp219.
[14] ROGUIN A,BEHAR D,BEN AMI H,et al. Long-term prognosis of acute pulmonary oedema--an ominous outcome[J]. Eur J Heart Fail,2000,2(2):137-144. doi:10.1016/s1388-9842(00)00069-6.
[15] HEIDENREICH P A,BOZKURT B,AGUILAR D,et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: Executive summary:A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines[J]. Circulation,2022,145(18):e876-e894. doi:10.1161/CIR.0000000000001106.
[16] 中华医学会心血管病学分会心力衰竭学组,中国医师协会心力衰竭专业委员会,中华心血管病杂志编辑委员会. 中国心力衰竭诊断和治疗指南2018[J]. 中华心血管病杂志,2018,46(10):760-789. Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association,Chinese Heart Failure Association of Chinese Medical Doctor Association,Editorial Board of Chinese Journal of Cardiology. Chinese guidelines for the diagnosis and treatment of heart failure 2018[J]. Chin J Cardiol,2018,46(10):760-789. doi:10.3760/cma.j.issn.0253-3758.2018.10.004.



