梁珊 何亚州 李丽娟
[摘要] 目的 利用生物信息学分析方法,探讨miRNA-494与慢性心力衰竭患者心肌纤维化机制的联系。 方法 利用GEO数据库筛选出GSE104150数据集,分析慢性心力衰竭患者及健康对照者差异表达miRNA,对其中miR-494预测靶基因及靶基因集分别进行基因本体(GO)富集分析及信号通路富集分析。 结果 与对照者比较,miR-494在慢性心力衰竭患者样本中呈差异性表达。在细胞质、细胞核等细胞组成,蛋白质结合等分子功能,细胞增殖与迁移、磷脂酰肌醇介导的信号传导等生物功能,以及多条心肌纤维化、慢性心力衰竭相关信号通路如转化生长因子-β、PI3K/Akt、MAPK等信号通路中富集。 结论 推测miR-494在慢性心力衰竭患者中差异性表达,并与心肌纤维化发生发展有一定相关性,有待进一步研究证实。
[关键词] miRNA;心肌纤维化;慢性心力衰竭;生物信息学
[中图分类号] R541.61 [文献标识码] A [文章编号] 1673-7210(2019)08(b)-0007-06
[Abstract] Objective To explore the mechanism of myocardial fibrosis in chronic heart failure patients by using bioinformatics analysis. Methods The GSE104150 dataset through GEO database was filtered out, the differentially expressed miRNAs between heart failure and healthy control was analyzed. The target genes of miR-494 were predicted, which should be performed the gene ontology (GO) enrichment analysis and signal pathway enrichment analysis. Results Compared with the healthy control, miRNA-494 was differentially expressed in the chronic heart failure patients. The GO analysis showed the target genes of miR-494 were enriched in cell components such as cytoplasm and nucleus, molecular functions as protein binding and biological functions as cell proliferation and migration, phosphatidylinositol-mediated signaling. The KEGG biological pathway was mainly enriched in TGFβ, PI3K/Akt, and MAPK signaling pathways. Conclusion It is speculated that miRNA-494 is differentially expressed in patients with chronic heart failure, and it has a certain correlation with the occurrence and development of myocardial fibrosis, further research is needed to confirm.
[Key words] MiRNA; Myocardial fibrosis; Chronic heart failure; Bioinformatics analysis
微小RNA(MicroRNA,miRNA)为非编码单链RNA分子。大量证据表明,其在癌症、心血管疾病等的发生发展过程中,常存在不同程度的异常表达[1-2]。研究发现,miR-208a与内皮糖蛋白表达及心脏纤维化相关,miRNA-221/222可能靶向与转化生长因子β(TGFβ)信号传导相关的几个基因[3-4]。
心肌纤维化心肌成纤维细胞(cardiac fibroblasts,CFs)异常增殖并分泌大量胶原蛋白,细胞外基质病理性积聚[5]。心力衰竭是由心肌梗死、心肌病等多种原因引起的心肌损伤,造成心肌结构和功能的变化,导致功能低下。慢性心力衰竭多合并心肌纤维化,心肌纤维化程度与心室射血分数关系密切,心力衰竭的标志性心肌损伤是心肌中间质和微血管周围内胶原纤维(即Ⅰ型胶原)的弥散积累[6-7]。有效防治心肌纤维化对慢性心力衰竭的发生发展有重大意义。基于此,本研究通过生物信息学分析,为防治慢性心力衰竭心肌纤维化提供新思路。
1 资料与方法
1.1 筛选差异性miRNA
使用Gene Expression Omnibus(GEO)数据库检索miRNA、慢性心力衰竭及心肌纤维化相关基因芯片。
1.2 miRNA靶基因预测及分析
使用TargetScanHuman、miRWalk、miRDB数据库,对miRNA靶基因进行检索。利用网页工具Bioinformatics & Evolutionary Genomics对总靶基因取交集处理。利用DAVID Bioinformatics Resources数据库工具和KOBAS网页工具对靶基因集进行功能富集分析及信号通路富集分析。
2 结果
2.1 差异性miRNA
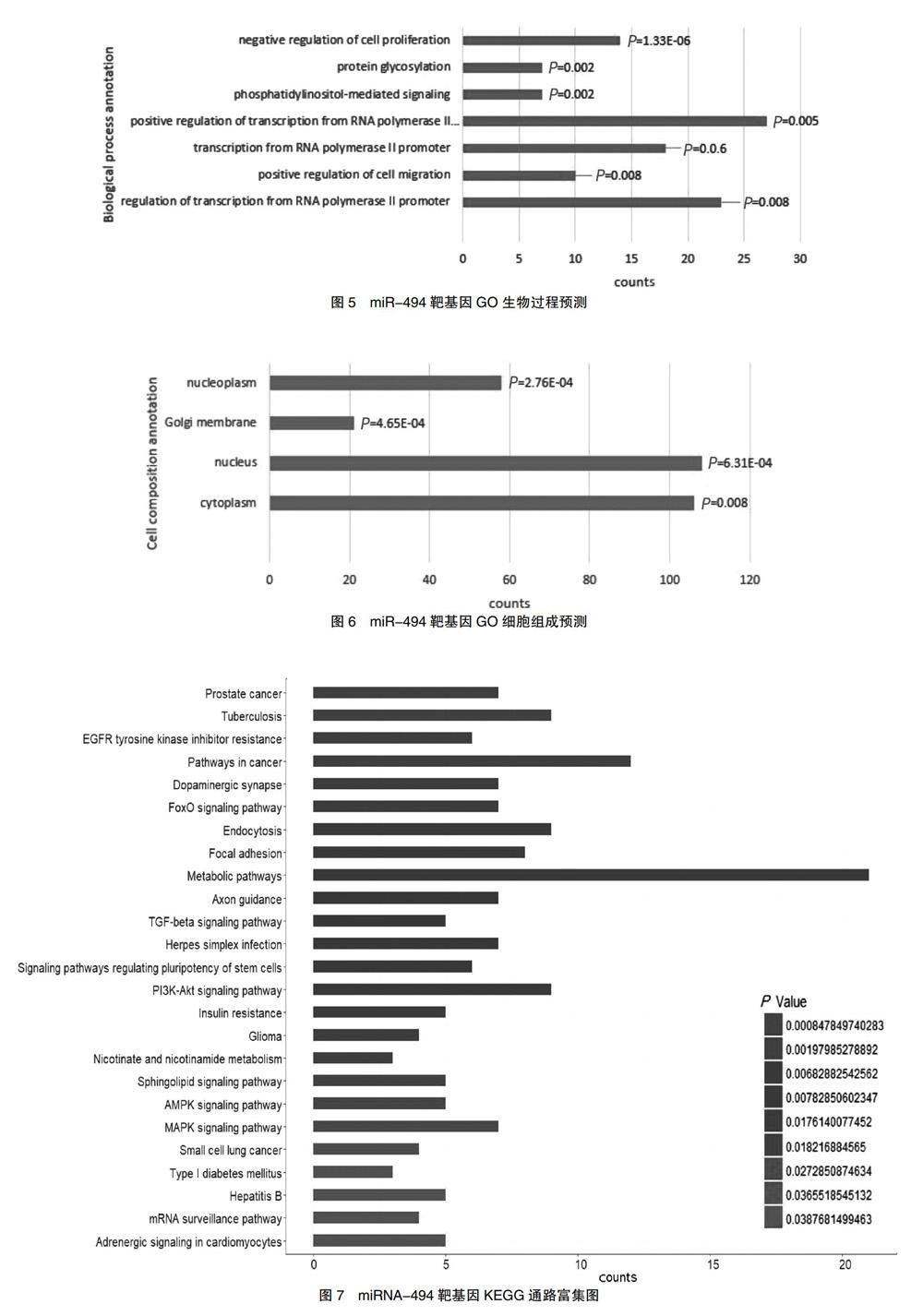
数据库检索并筛选出慢性心力衰竭与miRNA相关数据集GSE104150,其中包含人类健康对照及心力衰竭样本共16个,miRNA共2571个,将P值和logFC设定为:P < 0.01,logFC>2 or <-2,取前20个结果,绘制热图(图1,封四)。其中miRNA-494在慢性心力衰竭样本中呈高表达,见图2。经文献检索,miR-494在冠心病等心血管疾病中异常表达,并与慢性心力衰竭的发生发展相关。此外,miR-494在纤维化疾病中存在差异性表达,故选择miRNA-494作为进一步研究对象,探讨其在慢性心力衰竭心肌纤维化中的作用及影响。
2.2 miRNA-494靶基因预测
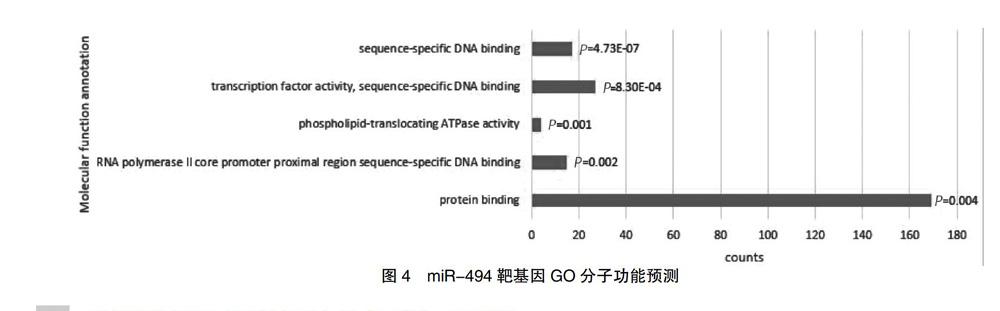
miRNA-494靶基因检索,TargetScan检索出2864条结果,miRWalk检索67 635条结果,miRDB检索607条结果,对上述数据库检索出的靶基因进行取交集,最终得到284个共同的靶基因。靶基因预测交集见图3。
2.3 miR-494靶基因功能预测
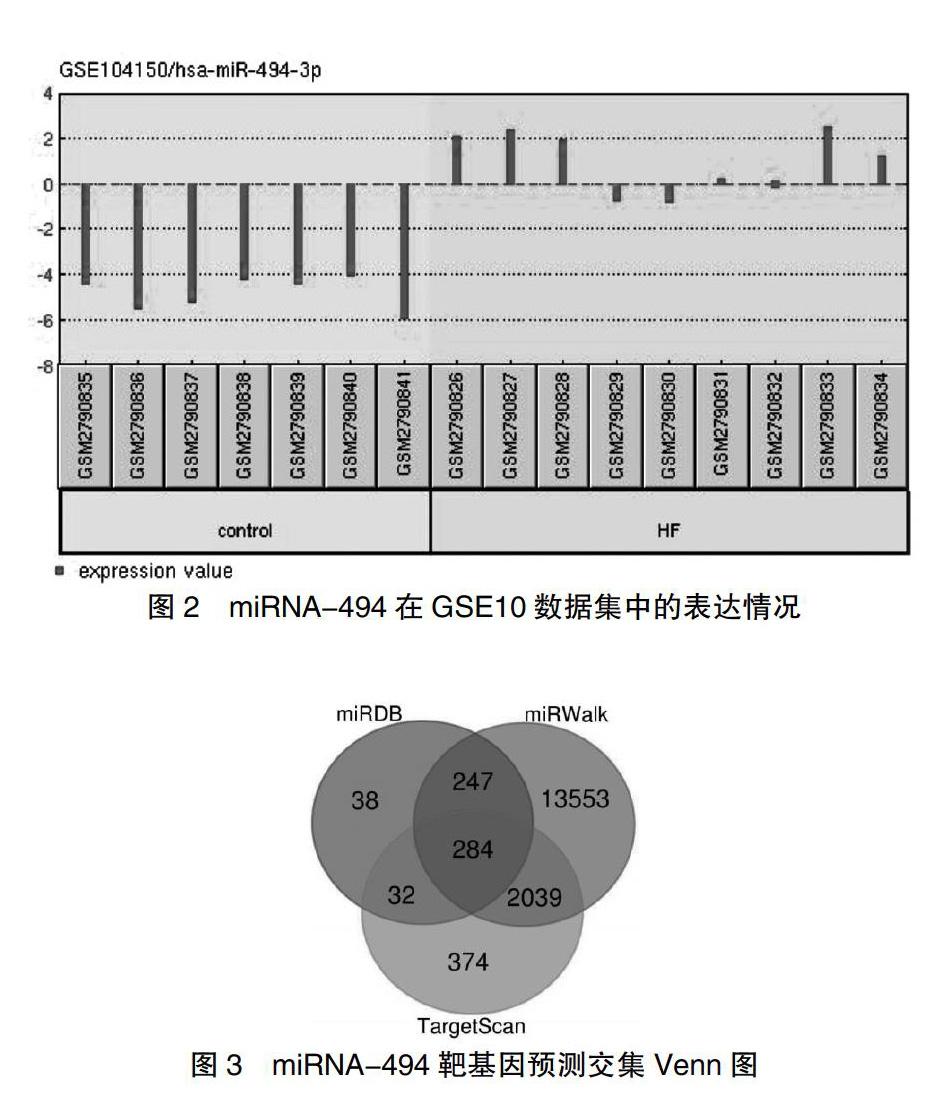
靶基因GO功能富集分析结果显示这些靶基因富集在蛋白质结合、RNA聚合酶Ⅱ核心启动子近端区序列特异性DNA结合、转录因子活性等分子功能中,在RNA聚合酶Ⅱ启动子调控转录、细胞迁移、磷脂酰肌醇介导的信号传导等生物过程中富集。并在细胞质、细胞核、高尔基膜、细胞内膜结合细胞器等细胞组成中富集(P < 0.01)。见图4~6。
2.4 靶基因KEGG通路预测
KOBAS 3.0网页靶基因集进行KEGG通路检索,发现miRNA-494靶基因在细胞代谢、肿瘤、鞘磷脂类信号通路、多能干细胞调节通路、TGFβ信号通路、MAPK信号通路信号传导等有关(P < 0.05)。见图7。
3 讨论
3.1 miRNA-494与慢性心力衰竭、心肌纤维化机制分析
心肌纤维化发生与发展机制涉及各种生长因子如TGFβ、基质金属蛋白酶(MMP)及其机制因子(TIMP),以及肾素-血管紧张素-醛固酮系统等[8-9]。研究表明MMP2、MMP9在压力负荷诱发心力衰竭、心肌纤维化过程中具有重要作用。而有研究表明miR-494的上调能抑制MMP9的表达[10]。M?觟hnle等[11]通过实验及计算分析发现miRNA-494能下调CB1受体,对心肌内源性大麻素系统产生影响,参与在心力衰竭的发展。
研究表明,miR-494与慢性心力衰竭相关的心肌内源性大麻素系统有关,并与心肌纤维化相关的基质金属蛋白酶系统有靶向关系,此外,结合本次数据分析结果发现miR-494与细胞增殖、分化与迁移相关。KEGG通路预测分析结果中miR-494涉及心肌纤维化的通路包括TGFβ、PI3K-Akt、AMPK、MAPK信号通路,且其中TGFβ、PI3K-Akt信号通路与心力衰竭的发展具有相关性[12-13]。由此可推测,miR-494与慢性心力衰竭心肌纤维化的发生发展相关。
3.2 miRNA-494通路预测分析
3.2.1 TGFβ信号通路 TGFβ信号通路被认为是组织修复和纤维化的公共通路,TGFβ超家族介导细胞的生长、增殖、分化,并在细胞外基质的形成等方面具有重要作用。KEGG通路分析发现,miR-494能靶向通路中的SARA、BMPRⅡ、p15等基因。研究发现,SARA的C端序列包含Smad结合结构域,能够抑制Smad2/3磷酸化并阻断Smad2/3和Smad4之间的相互作用,从而抑制纤维化。而p15基因,研究发现组蛋白去乙酰化酶抑制剂在心力衰竭的临床前模型中阻断心脏纤维化。研究[14-17]表明Ⅰ类蛋白去乙酰化酶抑制剂能通过抑制编码细胞周期蛋白依赖性激酶,阻断心脏成纤维细胞周期进展。此外,TGFβ除了促进心脏纤维化,也能激活反调节途径,用于调节心力衰竭中的TGF-β1活性[18]。
3.2.2 PI3K/Akt 信号通路 PI3K/Akt信号通路中,PI3K可调控Akt的激活,进一步激活或者抑制下游靶蛋白,参与多种细胞活动。KEGG通路分析,在PI3K/Akt通路中,miR-494靶向相关因子包括JAK、PTEN、PTK、PP2A等。Li等[19]敲除小鼠PP2A基因,发现PP2A活性的降低导致小鼠心肌细胞肥大和纤维化增加;而PP2ACα的缺失导致心力衰竭,表现为射血分数、FS、LV、心房利钠肽、脑利钠肽的变化;在分子层面上,基因敲除小鼠中Akt/GSK3β/β-连环蛋白途径的调节受到严重干扰。此外,Zhang等[20]的研究发现PP2A能在体外使细胞外调节蛋白激酶去磷酸化,从而阻断Ang Ⅱ刺激的α-SMA表达,即抑制心肌纤维化的发生。
3.2.3 MAPK信号通路 其组成因子能被细胞因子、激素等因素激活,调控细胞的生长、分化、应激适应、炎性反应等细胞过程[21]。KEGG通路分析,其涉及包括经典通路的RTK、RSK2以及JNK和p38/MAPK通路的LN1、JNK、JunD等。JNKs是MAPK家族的成员,介导炎症和细胞凋亡。有学者在研究抗氧化剂N-乙酰半胱氨酸(N-acetylcysteine,NAC)对长期升主动脉瓣狭窄大鼠心脏结构和功能的影响时发现,NAC能降低p-ERK和p-JNK蛋白表达,调控MAPK信号通路,减轻心肌纤维化,并降低右心室肥大的发生率[22-23]。
综上所述,此次分析显示miR-494在慢性心力衰竭患者及健康者之间差异性表达。其靶基因生物学功能预测结果涉及蛋白质结合、转录因子活性以及细胞迁移等,信号通路富集分析更与TGFβ信号通路、MAPK信号通路等心肌纤维化相关信号通路密切相关,可推测miR-494在慢性心力衰竭心肌纤维化发生发展过程中具有重要作用。miRNA的调控作用较为复杂,生物信息学方法分析、预测miRNA的靶基因及其功能等信息能够为后期实验提供依据,减少耗时及耗材。但由于生物信息分析因为算法不同有一定的局限性,因此在以生物信息学分析结果基础上仍需实验验证。
[参考文献]
[1] Huntzinger E,Izaurralde E. Gene silencing by microRNAs:contributions of translational repression and mRNA decay [J]. Nat Rev Genet,2011,12(2):99-110.
[2] Xu J,Shao T,Ding N,et al. miRNA-miRNA crosstalk:from genomics to phenomics [J]. Brief Bioinform,2017,18(6):1002-1011.
[3] Verjans R,Peters T,Beaumont FJ,et al. MicroRNA-221/222 Family Counteracts Myocardial Fibrosis in Pressure Overload-Induced Heart Failure [J]. Hypertension,2018, 71(2):280-288.
[4] Shyu KG,Wang BW,Cheng WP,et al. MicroRNA-208a Increases Myocardial Endoglin Expression and Myocardial Fibrosis in Acute Myocardial Infarction [J]. Can J Cardiol Can J Cardiol,2015,31(5):679-690.
[5] Qian W,Xin S,Rui C,et al. Ghrelin Ameliorates Angiotensin Ⅱ-Induced Myocardial Fibrosis by Upregulating Peroxisome Proliferator-Activated Receptor Gamma in Young Male Rats [J]. Biomed Res Int,2018,2018:1-14.
[6] Roy C,Slimani A,de Meester C,et al. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic resonance in heart failure with preserved ejection fraction [J]. J Cardiovasc Magn Reson,2018,20(1):55.
[7] Weber KT,Díez J. Targeting the Cardiac Myofibroblast Secretome to Treat Myocardial Fibrosis in Heart Failure [J]. Circ Heart Fail,2016,9(8):e003315.
[8] Heinzmann D,Fu?覻 S,Ungern-Sternberg SV,et al. TGFβ Is Specifically Upregulated on Circulating CD14++ CD16+ and CD14+ CD16++ Monocytes in Patients with Atrial Fibrillation and Severe Atrial Fibrosis [J]. Cell Physiol Biochem,2018,49(1):226-234.
[9] Iyer RP,Patterson NL,Fields GB,et al. The history of matrix metalloproteinases:milestones,myths,and misperceptions [J]. Am J Physiol Heart Circ Physiol,2012,303(8):H919-H930.
[10] Sun T,Cheung KSC,Liu ZL,et al. Matrix metallopeptidase 9 targeted by hsa-miR-494 promotes silybin-inhibited osteosarcoma [J]. Mol Carcinog,2018,57(2):262-271.
[11] M?觟hnle P,Schütz SV,Schmidt M,et al. MicroRNA-665 is involved in the regulation of the expression of the cardioprotective cannabinoid receptor CB2 in patients with severe heart failure [J]. Biochem Biophys Res Commun,2014,451(4):516-521.
[12] Heger J,Schulz R,Euler G,et al. Molecular switches under TGFβ signalling during progression from cardiac hypertrophy to heart failure [J]. Br J Pharmacol,2016,173(1):3-14.
[13] Li L,Zhao D,Jin Z,et al. Phosphodiesterase 5a Inhibition with Adenoviral Short Hairpin RNA Benefits Infarcted Heart Partially through Activation of Akt Signaling Pathway and Reduction of Inflammatory Cytokines [J]. PLoS One,2015,10(12):e0145766.
[14] Li SC,Ma LN,Chen J,et al. Effect of allicin on myocardial fibrosis after myocardial infarction in rats and its relationship with TGFβ/Smads signal transduction [J]. Zhongguo Zhong Yao Za Zhi,2016,41(13):2517-2521.
[15] Huang C,Du R,Zhang P,et al. Expression,purification,and functional characterization of recombinant PTD-SARA [J]. Acta Biochim Biophys Sin (Shanghai),2011, 43(2):110-117.
[16] Williams SM,Golden-Mason L,Ferguson BS,et al. Class I HDACs regulate angiotensin Ⅱ-dependent cardiac fibrosis via fibroblasts and circulating fibrocytes [J]. J Mol Cell Cardiol,2014,67:112-125.
[17] Liu M,Li Z,Liang B,et al. Hydrogen sulfide ameliorates rat myocardial fibrosis induced by thyroxine through PI3K/AKT signaling pathway [J]. Endocr J,2018,65(7):769-781.
[18] Morine KJ,Qiao X,York S,et al. Bone Morphogenetic Protein 9 Reduces Cardiac Fibrosis and Improves Cardiac Function in Heart Failure [J]. Circulation,2018,138(5):513-526.
[19] Li L,Fang C,Xu D,et al. Cardiomyocyte specific deletion of PP2A causes cardiac hypertrophy [J]. Am J Transl Res,2016,8(4):1769-1779.
[20] Zhang Y,Gao F,Tang Y,et al. Valproic acid regulates Ang Ⅱ-induced pericyte-myofibroblast trans-differentiation via MAPK/ERK pathway [J]. Am J Transl Res,2018,10(7):1976-1989.
[21] Lee J,Levin DE. Intracellular mechanism by which arsenite activates the yeast stress MAPK Hog1 [J]. Mol Biol Cell,2018,29(15):1904-1915.
[22] Tian J,Zhao Y,Liu Y,et al. Roles and Mechanisms of Herbal Medicine for Diabetic Cardiomyopathy:Current Status and Perspective [J]. Oxid Med Cell Longev,2017, 2017:8214541.
[23] Reyes DRA,Gomes MJ,Rosa CM,et al. N-Acetylcysteine Influence on Oxidative Stress and Cardiac Remodeling in Rats During Transition from Compensated Left Ventricular Hypertrophy to Heart Failure [J]. Cell Physiol Biochem,2017,44(6):2310-2321.
(收稿日期:2018-12-10 本文编辑:任 念)



