杨锐,索洁,李建锋,王文政,赵瑾,陶林,杨晓萍
实验研究
1,25(OH)2D3对Thy-1肾炎大鼠Ki67、mTOR表达的影响
杨锐1,索洁1,李建锋1,王文政1,赵瑾2,陶林2,杨晓萍3∆
目的研究1,25-二羟基维生素D3[1,25(OH)2D3]对Thy-1肾炎模型大鼠Ki67和哺乳动物雷帕霉素靶蛋白(mTOR)表达的影响,并探讨其机制。方法90只清洁级雄性SD大鼠随机分为对照组、模型组、1,25(OH)2D3组,每组30只。模型组与1,25(OH)2D3组尾静脉注射抗Thy-1单克隆抗体建立肾炎模型,对照组给予等剂量生理盐水。建模后,1,25(OH)2D3组给予1,25(OH)2D30.5 μg/d灌胃,连续给药21 d,对照组及模型组给予等体积花生油。分别于给药后第1、3、7、14、21天每组随机处死6只大鼠,处死前1 d收集24 h尿液进行24 h尿蛋白定量;取各组肾组织标本,经HE和PAS染色后进行肾脏病理损害评分,免疫组化法检测肾组织中Ki67、mTOR表达。结果模型组和1,25(OH)2D3组大鼠在建模后第1天尿蛋白水平升高,模型组第3天达高峰,至第14天恢复至正常水平,1,25(OH)2D3组大鼠第1、3、7天的尿蛋白水平均低于模型组(P<0.05)。1,25(OH)2D3组大鼠肾组织病理损害程度第3、7天较模型组减轻(P<0.05),Ki67、mTOR蛋白表达水平较模型组降低(P<0.05)。24 h尿蛋白定量,Ki67表达水平,mTOR表达水平及肾组织病理损害评分彼此间均呈正相关。结论1,25(OH)2D3可抑制Thy-1肾炎模型大鼠肾小球系膜细胞的增殖,其作用机制可能与减少Ki67、mTOR的表达有关。
维生素D;蛋白尿;哺乳动物雷帕霉素靶蛋白;Ki67;1,25(OH)2D3;Thy-1肾炎
肾小球肾炎是我国终末期肾脏病(ESRD)的首要病因,其发病的中心环节是系膜细胞的增殖和细胞外基质的异常沉积[1]。研究发现1,25-二羟基维生素D3[1,25(OH)2D3]可抑制系膜细胞增殖、诱导其凋亡[2]。磷脂酰肌醇-3激酶(PI3K)/蛋白激酶(Akt)/哺乳动物雷帕霉素靶蛋白(mTOR)信号转导通路的活化可促进细胞的增殖、肥大[3]。然而mTOR信号转导通路在1,25(OH)2D3调控系膜细胞过程中的作用尚不清楚。本研究拟通过建立Thy-1肾炎大鼠模型,探讨1,25(OH)2D3对大鼠肾组织Ki67和mTOR的表达的影响及机制。
1 材料与方法
1.1 动物及试剂90只清洁级雄性SD大鼠,体质量(182.30±13.17)g,购自新疆地方流行性疾病控制中心。抗Thy-1单克隆抗体购自Cedarlane公司。兔抗鼠Ki67单克隆抗体和兔抗鼠mTOR单克隆抗体购自Cell Signaling公司。1, 25(OH)2D3购自上海罗氏制药有限公司(0.25 μg/粒)。花生油购自山东鲁花集团有限公司。Envision免疫组化试剂盒、二氨基联氨苯(DAB)显色试剂盒购自北京金桥生物工程有限公司。
1.2 Thy-1肾炎模型制备及干预按随机数字表法将90只大鼠分为对照组、模型组、1,25(OH)2D3组,每组30只。各组大鼠适应性喂养1周后,模型组和1,25(OH)2D3组一次性尾静脉注射抗Thy-1单克隆抗体,注射剂量为25 μL/100 g[4],对照组大鼠给予等剂量生理盐水。模型建立后1,25(OH)2D3组每日给予1,25(OH)2D30.5 μg溶于1 mL花生油中灌胃,连续给药21 d。对照组和模型组灌胃等体积花生油。
1.3 标本收集与处理分别于给药干预后第1、3、7、14、21天,各组随机处死动物6只。留取肾组织,经4%多聚甲醛固定、石蜡包埋。处死前1 d收集24 h尿液,考马斯亮蓝法进行24 h尿蛋白定量。
1.4 肾组织病理损害观察将已固定的肾进行组织修复(大小约为1.0 cm×1.0 cm×0.5 cm)、乙醇梯度脱水、二甲苯透明及石蜡包埋后进行切片,厚度约3 μm。苏木素和伊红(HE)染色、1%过碘酸溶液和苏木素(PAS)染色,封片后在光镜下观察肾小球系膜细胞和基质增生情况,参照人系膜增生性肾炎分级标准进行病理损害程度分级评分[5]。
1.5 Ki67和mTOR表达检测采用免疫组化二步法,具体操作按Envision检测试剂盒说明书进行。Ki67抗体工作浓度1∶800,mTOR抗体工作浓度1∶50,以磷酸盐缓冲液(PBS)代替一抗作为阴性对照,染色后DAB显色,中性树胶封片。于高倍镜下(×400)随机读取相对完整的5个肾小球,观察其染色强度和阳性细胞百分比,以两者的积分值乘积确定其表达水平[6]。染色强度评分:无色计0分,淡黄色计1分,棕黄色计2分,棕褐色计3分(染色深浅需与背景着色相对比)。阳性细胞百分比评分:阴性计0分,<10%计1分,11%~50%计2分,51%~75%计3分,>75%计4分。
1.6 统计学方法采用SPSS 20.0统计软件进行数据处理,计量资料采用表示,多组间均数比较采用单因素方差分析,组间多重比较用LSD-t法,相关性分析采用Pearson相关,P<0.05为差异有统计学意义。
2 结果
2.1 各组24 h尿蛋白定量结果模型组大鼠建模后第1天尿蛋白水平升高,第3天达到峰值,均高于同期对照组(P<0.05),至第14天恢复正常水平。1,25(OH)2D3组蛋白尿水平在第1、3、7天低于模型组(P<0.05),见表1。
Tab.1Twenty four hour urinary protein quantitation in each group表1 各组24 h尿蛋白定量结果(n=6,mg/d,)
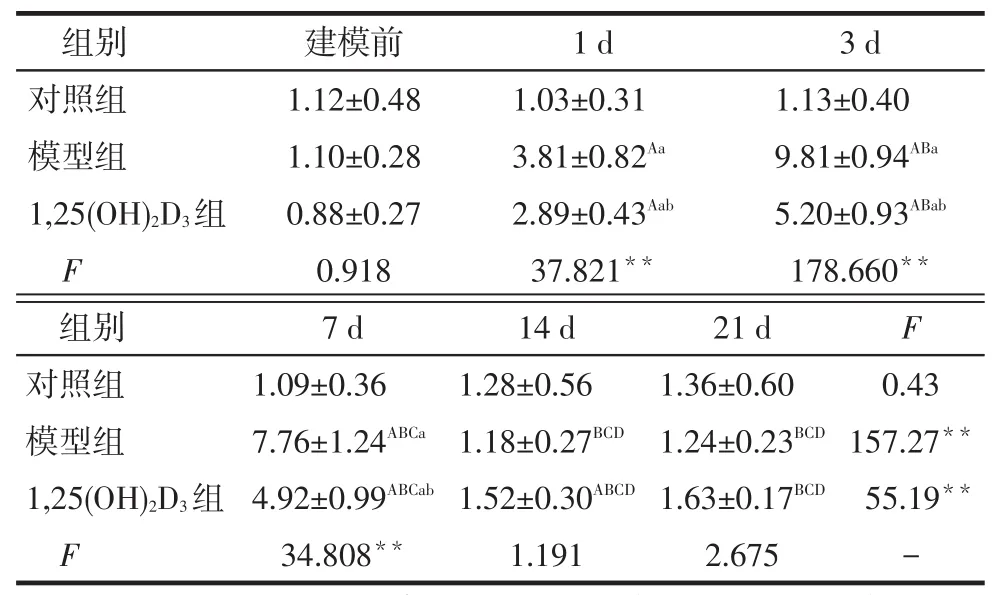
Tab.1Twenty four hour urinary protein quantitation in each group表1 各组24 h尿蛋白定量结果(n=6,mg/d,)
**P<0.01;组内比较:A与建模前相比,B与第1天相比,C与第3天相比,D与第7天相比,P<0.05;组间比较:a与对照组相比,b与模型组相比,P<0.05。
组别对照组模型组1,25(OH)2D3组F建模前1.12±0.48 1.10±0.28 0.88±0.27 0.918 1 d 1.03±0.31 3.81±0.82Aa 2.89±0.43Aab 37.821**3 d 1.13±0.40 9.81±0.94ABa 5.20±0.93ABab 178.660**F 0.43 157.27**55.19**-组别对照组模型组1,25(OH)2D3组F 7 d 1.09±0.36 7.76±1.24ABCa 4.92±0.99ABCab 34.808**14 d 1.28±0.56 1.18±0.27BCD 1.52±0.30ABCD 1.191 21 d 1.36±0.60 1.24±0.23BCD 1.63±0.17BCD 2.675
2.2 各组大鼠肾组织病理损害比较对照组大鼠肾组织未见系膜细胞和基质增生;模型组可见系膜细胞增生,毛细血管袢受压严重,出现结节和团块状实性区,部分肾小球出现分叶、硬化和纤维化;1,25(OH)2D3组可见毛细血管袢轻度受压,系膜宽度未超过毛细血管直径,呈节段性分布,见图1。模型组于建模后第7天系膜细胞增生程度明显,此后逐渐减弱,1,25(OH)2D3组在第3、7天系膜细胞增生程度较模型组减轻(P<0.05),见表2。
2.3 Ki67和mTOR表达结果Ki67表达阳性细胞呈棕褐色颗粒状,位于细胞核,偶见于正常的大鼠肾小球系膜细胞,见图2。建模后模型组大鼠第1天即出现水平升高,于第3天达到峰值,此后逐渐恢复至正常水平。1,25(OH)2D3组第1、7天肾组织Ki67表达水平较模型组降低(P<0.05),见表3。
Tab.2Comparison of renal pathological damage scores in different groups表2 各组肾组织病理损害评分结果比较(n=6,分,)
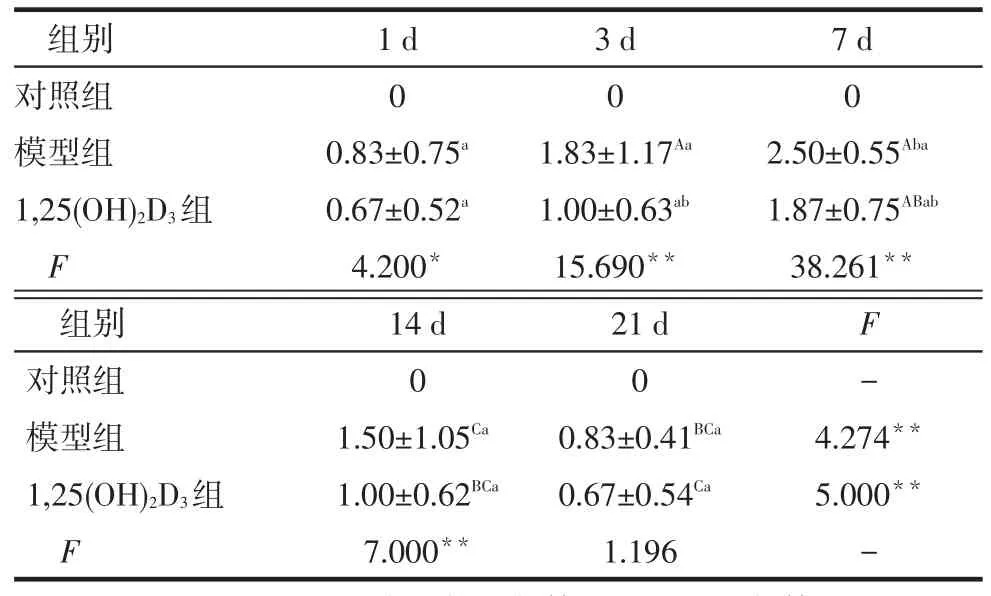
Tab.2Comparison of renal pathological damage scores in different groups表2 各组肾组织病理损害评分结果比较(n=6,分,)
*P<0.05,**P<0.01;组内比较:A与第1天相比,B与第3天相比,C与第7天相比,P<0.05;组间比较:a与对照组相比,b与模型组相比,P<0.05,表3、4同
组别对照组模型组1,25(OH)2D3组F 1 d 0 0.83±0.75a 0.67±0.52a 4.200*3 d 0 1.83±1.17Aa 1.00±0.63ab 15.690**7 d 0 2.50±0.55Aba 1.87±0.75ABab 38.261**F-4.274**5.000**-组别对照组模型组1,25(OH)2D3组F 14 d 0 1.50±1.05Ca 1.00±0.62BCa 7.000**21 d 0 0.83±0.41BCa 0.67±0.54Ca 1.196
Tab.3Comparison expressions of Ki67 in different groups表3 各组肾组织Ki67表达评分比较(n=6,分)
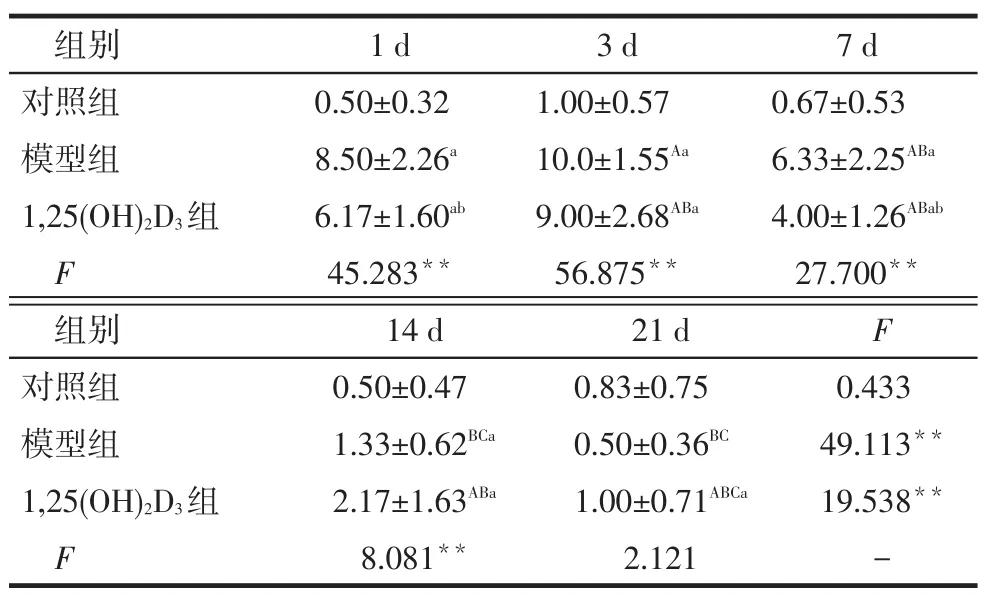
Tab.3Comparison expressions of Ki67 in different groups表3 各组肾组织Ki67表达评分比较(n=6,分)
组别对照组模型组1,25(OH)2D3组F 1 d 0.50±0.32 8.50±2.26a 6.17±1.60ab 45.283**3 d 1.00±0.57 10.0±1.55Aa 9.00±2.68ABa 56.875**7 d 0.67±0.53 6.33±2.25ABa 4.00±1.26ABab 27.700**组别对照组模型组1,25(OH)2D3组F 14 d 0.50±0.47 1.33±0.62BCa 2.17±1.63ABa 8.081**21 d 0.83±0.75 0.50±0.36BC 1.00±0.71ABCa 2.121 F 0.433 49.113**19.538**-
mTOR在系膜细胞中的阳性表达呈巢状、条索状或者是弥散分布的棕黄色颗粒,位于细胞核,见图3。建模后模型组大鼠肾组织mTOR表达水平逐渐升高,于建模后第7天达到峰值,此后逐渐减弱;1, 25(OH)2D3组第3、7、14天mTOR表达水平较模型组降低(P<0.05),见表4。
2.4 各指标相关性分析24 h尿蛋白定量、Ki67表达水平、mTOR表达水平之间均呈正相关,且3者均与病理损害分级评分呈正相关,见表5。
Tab.4Comparison expression of mTOR in different groups表4 各组肾组织mTOR表达评分比较(n=6,分,)
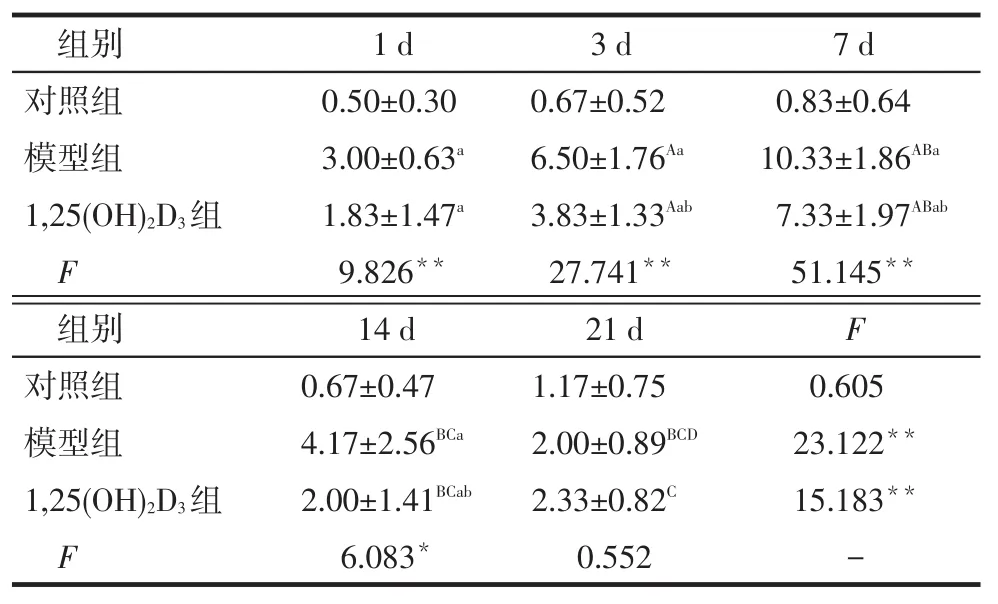
Tab.4Comparison expression of mTOR in different groups表4 各组肾组织mTOR表达评分比较(n=6,分,)
组别对照组模型组1,25(OH)2D3组F 1 d 0.50±0.30 3.00±0.63a1.83±1.47a9.826**3 d 0.67±0.52 6.50±1.76Aa3.83±1.33Aab27.741**7 d 0.83±0.64 10.33±1.86ABa7.33±1.97ABab51.145**组别对照组模型组1,25(OH)2D3组F 14 d 0.67±0.47 4.17±2.56BCa2.00±1.41BCab6.083*21 d 1.17±0.75 2.00±0.89BCD2.33±0.82C0.552 F 0.605 23.122**15.183**-

Tab.5Relationship between histopathological grading of renal damage,Ki67 and mTOR in glomerular表5 在肾小球中肾组织病理损害分级评分、Ki67、mTOR之间的关系(r)
3 讨论
Thy-1肾炎模型诱导成功率高,且试验周期短,已被广大研究者接受和认可。本研究中,Thy-1肾炎模型从临床指标和组织学形态方面均与国内外报道一致[7],可满足研究需要。
系膜细胞是肾小球的固有细胞之一,其异常增殖及继发的炎症介质释放、细胞外基质的病理性集聚是导致肾小球硬化、促使各种肾小球肾炎向终末期肾病发展的中心环节。因此,维持系膜细胞增生、肥大与凋亡之间的平衡及改善系膜基质代谢是延缓或逆转肾小球硬化的关键。大量研究已经证实1,25(OH)2D3可抑制系膜细胞增殖[2,8-9]。本研究立足于此结论,着重于1,25(OH)2D3对于系膜细胞作用靶点的研究。
本研究发现模型组大鼠在建模后第1天即出现尿蛋白水平升高,第3天达高峰,至第14天恢复正常水平,这可能与Thy-1肾炎模型有自愈倾向有关。1,25(OH)2D3组大鼠蛋白尿水平在第1、3、7天均低于模型组,提示1,25(OH)2D3可减少蛋白尿,对肾脏具有保护作用,与Szeto等[10]研究结果一致。1,25(OH)2D3组大鼠在第3、7天的病理损害改变较模型组明显减轻,提示1,25(OH)2D3对于Thy-1肾炎的调控不仅是在减少蛋白尿方面,而且也体现在组织形态学改变方面。
Ki67作为评价细胞增殖状态的一个指标,其表达于除G0期以外的各增殖细胞周期,近年来被广泛用于测定各种肿瘤的增殖活性,如乳腺癌等[11]。本课题组前期研究发现,在Thy-1大鼠肾炎肾组织系膜细胞中,增殖细胞核抗原(PCNA)的表达明显增高[12]。本研究结果显示,模型组及1,25(OH)2D3组大鼠肾组织系膜细胞中存在Ki67表达增高,提示Ki67及PCNA均可作为大鼠系膜细胞增殖的检测
指标。1,25(OH)2D3组在第1,7天较模型组Ki67的表达减少,表明1,25(OH)2D3可抑制Ki67的表达,进而抑制系膜增生性肾小球肾炎的进展。
mTOR是细胞存活、增殖及凋亡过程中的关键蛋白,在多种肿瘤细胞中存在mTOR的持续激活[13]。有研究发现小鼠狼疮性肾炎中mTOR表达上调,且系膜细胞的病理性增殖与其相关[3]。同时已经证实1,25(OH)2D3可诱导多种细胞分化并抑制其增殖[14],那么其生物学作用的发挥是否通过mTOR介导?Regulska等[15]发现mTOR参与了1,25(OH)2D3及其类似物对神经细胞的保护作用。1,25(OH)2D3及其受体可抑制星状孢子素介导的成骨细胞凋亡,其过程同样有mTOR的参与[16]。在本研究中,模型组及1,25(OH)2D3组大鼠肾组织中mTOR的表达水平较对照组升高,1,25(OH)2D3组大鼠mTOR的表达在第3、7、14天较模型组降低,且mTOR的表达与细胞周期调节蛋白Ki67的表达呈正相关,说明1,25(OH)2D3可抑制mTOR的表达,从而抑制系膜细胞的增殖。至于1,25(OH)2D3抑制mTOR表达的具体机制,尚需从分子生物学方面进行深入研究。
(图1~3见插页)
[1]Wei X,Li Z,Chen W,et al.Prevalence and risk factors of chronic kidney disease in first-degree relatives of chronic kidney disease patients in Southern China[J].Nephrology(Carlton),2012,17(2): 123-130.doi:10.1111/j.1440-1797.2011.01523.x.
[2]Yang XP,Chen GX,Xu G,et al.Active vitamin D3 on mesangial cell growth cycle and apoptosis[J].Chinese Journal of Kidney Dis⁃ease,2008,24(6):443-444.[杨晓萍,陈桂香,徐钢,等.活性维生索D3对肾小球系膜细胞生长、周期及凋亡的影响[J],中华肾脏病杂志,2008,24(6):443-444].doi:10.3321/i.issn:1001-7097.2008.0 6.014.
[3]Follo MY,Manzoli L,Poli A,et al.PLC and PI3K/Akt/mTOR signal⁃ling in disease and cancer[J].Adv Biol Regul,2015,57:10-16.doi: 10.1016/j.jbior.2014.10.004.
[4]Hashimoto K,Kamijo Y,Nakajima T,et al.PPARα activation pro⁃tects against anti-Thy1 nephritis by suppressing glomerular NF-κB signaling[J].PPAR Res 2012,10(10):1-11.doi:10.1155/2012/ 976089.
[5]Zhan YP.Mesangial proliferative glomerulonephritis.Wang HY edi⁃tor.Nephrology[M].3rd edition.Beijing:People's Medical Publish⁃ing House,2008:1016-1023.[湛贻璞.系膜增生性肾炎.王海燕.肾脏病学[M].第3版.北京:人民卫生出版社,2008:1016-1023].
[6]Fan ZJ,Yang FL,Zhu GC.Significance of expression of β-catenin and VEGF in hepatocellular carcinoma[J].World Chinese Journal of Digestology,2015,23(4):665-670.[范正军,杨飞龙,朱广灿.β-连环素和血管内皮生长因子在肝细胞癌中的表达及意义[J].世界华人消化杂志,2015,23(4):665-670].doi:10.11569/wcjd.v23. i4.665.
[7]Qin D,Morita H,Inui K,et al.Aldosterone mediates glomerular in⁃flammation in experimental mesangial proliferative glomerulone⁃phritis[J].J Nephrol,2013,26(1):199-206.doi:10.5301/ jn.5000125.
[8]Lucisano S,Buemi M,Passantino A,et al.New insights on the role of vitamin D in the progression of renal damage[J].Kidney&Blood Pressure Research,2013,37(6):667-678.doi:10.1159/000355747.
[9]Yin X,Zhang H,Cheng JP,et al.Effects of 1,25(OH)2D3on prolifer⁃ation and expression of PCNA of human glomerular mesangial cells[J].Tianjin Med J,2015,43(1):17-20.[尹漩,张昊,陈建平,等.1,25(OH)2D3对人肾小球系膜细胞增殖及PCNA表达的影响[J].天津医药,2015,43(1):17-20].doi:10.3969/j.issn.0253-9896.
[10]Szeto CC,Chow KM,Kwan BC,et al.Oral calcitriol for the treat⁃ment of persistent proteinuria in immunoglobulin A nephropathy:an uncontrolled trial[J].Am J Kidney Dis,2008,51(5):724-731.doi: 10.1053/j.ajkd.2007.12.038.
[11]Shui R,Yu B,Bi R,et al.An interobserver reproducibility analysis of ki67 visual assessment in breast cancer[J].PLoS One,2015,10(5):e0125131.doi:10.1371/journal.pone.0125131.
[12]Nie YF,Liu LR,Yang XP,et al.Affect the activity of vitamin D3 on rat mesangial proliferative glomerulonephritis and PCNA[J].Prog⁃ress in Modern Biomedicine,2009,9[10]:1979-1983.[聂艳芳,刘丽蓉,杨晓萍,等.活性维生索D3对大鼠系膜增生性肾炎PCNA的影响[J].现代生物医学进展,2009,9(10):1979-1983].
[13]Cao Y,Liu X,Lu W,et al.Fibronectin promotes cell proliferation and invasion through mTOR signaling pathway activation in gallbladder cancer[J].Cancer Lett,2015,360(2):141-150.doi:10.1016/j.canlet.
[14]Koike H,Morikawa Y,Sekine Y,et al.Survivin is associated with cell proliferation and has a role in 1a,25-dihydroxyvitamin D3 in⁃duced cell growth inhibition in prostate cancer[J].J Urol,2011,185(4):1497-1503.doi:10.1016/j.juro.2010.12.005.
[15]Regulska M,Leoekiewvicz M,Budziszewska B,et al.Inhibitory ef⁃fects of 1,25-dihydroxyvitamin D3 and its low-calcemic analogues on staurosporine-induced apoptosis[J].Pharmacol Rep,2007,59(4):393-401.
[16]Zhang X,Zanello LP.Vitamin D recepter-dependent 1 alpha,25(OH)2 vitamin D3-Induced Anti-Apoptotic PI3K/Akt signaling in Osteo⁃blasts[J].Bone Miner Res,2008,23(8):1238-1248.doi:10.1359/jbmr. 080326.
(2015-03-17收稿 2015-05-23修回)
(本文编辑 胡小宁)
Effect of 1,25-dihydroxyvitamin D3influence on expressions of Ki67 and mTOR in Thy-1 nephritis model of rat
YANG Rui1,SUO Jie1,LI Jianfeng1,WANG Wenzheng1,ZHAO Jin2,TAO Lin2,YANG Xiaoping3∆
1 College of Medicine,Shihezi University,Xinjiang 832000,China;2 Department of Pathology,School of Medicine,Shihezi University;3 Department of Nephrology,the First Affiliated Hospital,School of Medicine,Shihezi University∆
ObjectiveTo study the expressions of Ki67 and mTOR in Thy-1 nephritis model of rat who were given 1,25-dihydroxyvitamin D3[1,25(OH)2D3]and to explore its mechanism.MethodsHealthy male SD rats(n=90)were random⁃ly divided into three groups:control group,model group,1,25(OH)2D3treatment group(n=30 in each group).Model group and 1,25(OH)2D3treatment group were intravenously injected with anti-Thy1 monoclonal antibody once via tail vein while the control group were administrated with same volume of normal saline through the same route.1,25(OH)2D3were adminis⁃tratedat 0.5 μg per day intra-gastrically for consecutive 21 days in 1,25(OH)2D3treatment group while equal volume of pea⁃nut oil were given in control group and model group.Six rats were randomly selected from each group and sacrificed at the 1st,3rd,7th,14thand 21stafter drug intervention.Twenty four hour urine sample were collected in each rat just before it was culled to detect 24-hour urinary protein excretion.Renal tissue samples were harvested and stained with hematoxylin&eo⁃sin(H&E)and PAS to determine the renal pathological variation and the expressions of mTOR and Ki67 were assessed by immunohistochemistry.ResultsUrine protein begin to be detected at the first day after model was established,peaked at the 3rddays then started dropping until the 14thday when urine sample turned to normal.Urine protein levels were lower in 1, 25(OH)2D3treatment group at the 1st,3rd,7thday after model establishment than those in model group(P<0.05).Compared with model group,the pathological damage of renal tissue in 1,25(OH)2D3treatment group were alleviated at the 3rdand 7thday after model establishment(P<0.05).Expressions of Ki67 and mTOR in 1,25(OH)2D3treatment group were reduced compared with those in model group(P<0.05).Twenty four hour urinary protein and expressions of Ki67 and mTOR as well as renal pathological damage were all positively correlated with each other.Conclusion1,25(OH)2D3can inhibit the proliferation of glomerular mesangial cells in Thy-1 nephritis model of rat.And its therapeutic mechanism may be associated with down reg⁃
vitamin D;proteinuria;mTOR;Ki67;1,25(OH)2D3;Thy-1 nephritis
R692
A
10.11958/j.issn.0253-9896.2015.10.007
国家自然科学基金资助项目(81160090)
1新疆石河子大学医学院(邮编832000);2新疆石河子大学医学院病理教研室;3新疆石河子大学医学院第一附属医院肾病科
杨锐(1988),男,硕士研究生,主要从事肾小球疾病及其发病机制研究
∆通讯作者E-mail:sbkyxp@163.com
ulating expressions of Ki67 and mTOR.



