齐艳,赵秀娟,徐琳琪,吴旭东△,汪建涛
细胞与分子生物学
白藜芦醇对H2O2及TGF-β2诱导人小梁网细胞的影响及机制探讨
齐艳1,2,赵秀娟2,徐琳琪1,2,吴旭东2△,汪建涛1△
目的 观察过氧化氢(H2O2)及转化生长因子(TGF)-β2诱导人小梁网细胞(HTMCs)后对纤维连接蛋白(FN)、胶原蛋白1型(COL1)、核因子(NF)-κB P65蛋白和白细胞介素(IL)-1β基因表达的影响及白藜芦醇(RSV)的干预作用。方法 (1)选取汇合度70%~80%的HTMCs分为5组。实验组于无血清培养基中分别加入浓度为150、300、450、800 μmol/L的H2O2处理,对照组的培养基中不加H2O2。Western blot法检测各组FN、COL1、NF-κB P65、NF-κB P65磷酸化(P-NF-κB P65)蛋白的表达,实时定量PCR法检测IL-1β基因的表达。(2)HTMCs细胞分为3组。对照组以不含H2O2及RSV的无血清培基处理,H2O2组以300 μmol/L的H2O2处理,H2O2+RSV组同时加入300 μmol/L的H2O2及25 μmol/L的RSV处理。检测各组上述蛋白和基因的表达情况。免疫荧光检测各组NF-κB P65 在HTMCs中的定位。(3)HTMCs细胞分为3组。对照组以不含TGF-β2及RSV的无血清培基处理,TGF-β2组以5 μg/L的TGF-β2处理,TGF-β2+RSV组为同时加入5 μg/L的TGF-β2及25 μmol/L的RSV处理。检测各组上述蛋白和基因的表达情况。结果 (1)与对照组比较,150、300、450、800 μmol/L组FN和P-NF-κB P65蛋白表达水平均增高,300、450、800 μmol/L组COL1蛋白和IL-1β基因表达水平增高(P<0.05),其他指标比较差异均无统计学意义。(2)H2O2组较对照组FN、COL1、P-NF-κB P65蛋白和IL-1β基因表达水平均增高,而H2O2+RSV组较H2O2组上述指标均降低,H2O2+RSV组较对照组仅IL-1β降低(P<0.05)。对照组仅细胞质表达NF-κB P65,H2O2组细胞胞质及核中均有NF-κB P65表达,且核中表达较多;H2O2+RSV组细胞胞质中表达NF-κB P65较核中多。(3)TGF-β2组较对照组FN、COL1、P-NF-κB P65的蛋白和IL-1β基因水平表达均增高(P<0.05),TGF-β2+RSV组较TGF-β2组上述指标均降低(P<0.05)。结论 H2O2和TGF-β2能上调HTMCs的FN、COL1、P-NF-κB P65蛋白及IL-1β基因的表达,可能参与青光眼的发生发展过程。RSV能抑制H2O2和TGF-β2对HTMCs的影响,对青光眼发挥一定的保护作用。
青光眼;小梁网;过氧化氢;转化生长因子β2;细胞外基质;核因子-κB;白细胞介素1β;纤维连接蛋白;胶原蛋白1型;白藜芦醇
青光眼在全球致盲眼病中居第2位,严重威胁患者的视觉质量[1]。小梁网细胞(trabecular meshwork cells,TMCs)细胞外基质(extracellular matrix,ECM)的异常沉积可使房水流出阻力增加,引起眼压升高,这是开角型青光眼(open angle glaucoma,OAG)的主要发病机制之一[2]。氧化应激、转化生长因子(transforming growth factor,TGF)-β2等可引起TMCs的ECM异常沉积[3-5]。其中,氧化应激还可通过激活核因子(nuclear factor,NF)-κB,使多种炎性因子表达升高[6]。研究表明,OAG患者小梁网组织中炎性因子白细胞介素(IL)-1β表达升高[7]。因此,青光眼的发生发展也可能与炎症的发生有关。白藜芦醇(resveratrol,RSV)是非黄酮类多酚化合物,具有较强的抗氧化能力[8]。研究表明,RSV可抑制TMCs中活性氧的生成[9]及多种炎性因子的表达[10],但RSV能否抑制TMCs的ECM异常增多尚少见相关报道。本研究通过观察RSV对H2O2及TGF-β2刺激下TMCs的ECM、NF-κB P65 及IL-1β的影响,探讨RSV对青光眼的保护作用及可能机制。
1 材料与方法
1.1 材料 人小梁网细胞株(human trabecular meshwork cells,HTMCs)购自美国ScienCell实验室;过氧化氢(H2O2)购自中国aladdin公司;TGF-β2购自美国Peprotech公司;RSV购自美国Sigma公司;DMEM细胞培养液、胎牛血清、L-谷氨酰胺、丙酮酸钠、非必需氨基酸、青霉素/链霉素均购自美国Gibco公司。纤维连接蛋白(Fibronectin,FN)抗体、胶原纤维1型(Collagen 1,COL1)抗体均购自英国Abcam公司,NF-κB P65磷酸化(P-NF-κB P65)抗体、NF-κB P65抗体均购自美国CST公司,β-actin抗体购自南京诺唯赞生物科技公司;Western blot用羊抗鼠二抗及羊抗兔二抗购自美国KPL公司,免疫荧光用罗丹明标记山羊抗兔二抗购自北京中杉金桥公司;DAPI购自北京索莱宝公司,Trizol试剂及逆转录试剂盒购自美国Thermo公司;PVDF转印膜购自瑞士Roche公司,Bradford蛋白定量试剂购自美国BIO-RAD公司;ECL试剂盒购自美国PerkinElmer公司。
1.2 细胞培养 HTMCs培养基为含10%胎牛血清、20 mmol/L L-谷氨酰胺、1 mmol/L丙酮酸钠、100 μmol/L非必需氨基酸、100 U/mL青霉素、100 mg/L链霉素的DMEM高糖培养基,置于37℃含5%CO2的细胞培养箱培养。细胞生长状态良好,待汇合度达70%~80%后用于后续实验。
1.3 不同浓度H2O2对HTMCs的处理 选取汇合度70%~80%的HTMCs,在无血清培养基中分别加入浓度为150、300、450、800 μmol/L的H2O2处理2 h,另设不加H2O2处理HTMCs培养2 h为对照组。
1.3.1 Western blot法检测FN蛋白、COL1蛋白、NF-κB P65蛋白、P-NF-κB P65蛋白的表达水平 不同浓度H2O2处理HTMCs 2 h后,收集细胞,用含蛋白酶抑制剂(PMSF)的RIPA裂解液(25 mmol/L Tris-HCl pH=7.6,5 mol/L NaCl,1%TritonX-100,1% Na-deoxycholate,0.1%SDS,1 mmol/L EDTA)裂解HTMCs,冰上操作提取蛋白。用Bradford试剂进行蛋白定量后,取20~40 μg总蛋白进行SDS-PAGE电泳。分离胶总丙烯酰胺百分浓度选为8%(W∶V)。电泳结束后,采用湿转(4℃,300 mA,2 h)将蛋白转至PVDF膜上,室温封闭1 h,一抗4℃孵育过夜(FN抗体1∶8 000,COL1抗体1∶5 000,NF-κB P65抗体1∶3 000,P-NF-κB P65抗体1∶1 000,β-actin抗体1∶2 000)。次日,经TBST漂洗3次后,室温孵育二抗1 h(羊抗兔、羊抗鼠抗体1∶1 000)。TBST漂洗3次后,在PVDF膜上滴加ECL发光试剂,放入凝胶成像仪自动成像系统曝光。以β-actin为内参,Western blot法检测FN蛋白、COL1蛋白、NF-κB P65蛋白、P-NF-κB P65蛋白的表达。
1.3.2 实时定量PCR法检测IL-1β基因的表达 不同浓度H2O2处理HTMCs 2 h后,收集细胞,按照试剂盒说明书用Trizol试剂提取细胞总RNA,取吸光度比值(A260/280)在1.8~2.0之间的总RNA(1 μg)进行实验。先使用DNase I去除可能的DNA污染后进行逆转录合成cDNA。以cDNA为模板在7500实时荧光定量PCR仪上进行qPCR扩增。引物序列(5′→3′)GAPDH:上游GCACCGTCAAGGCTGAGAAC;下游TGGTGAAGACGCCAGTGGA;IL-1β:上游AACCTCTTCGAG GCACAAGG;下游GGCGAGCTCAGGTACTTCTG;GAPDH和IL-1β扩增长度分别为138和107 bp。反应体系:模板cDNA 1 μL,10 μmol/L上、下游引物各 0.1 μL,2×SYBR GREEN Master Mix 5 μL,无RNA酶水补足反应体系至10 μL。反应条件:95℃预变性5 min;95℃变性15 s,60℃退火和延伸40 s,40个循环。选取GAPDH作为内参基因。2-ΔΔCt法比较相对表达量,ΔΔCt=样本ΔCt-对照ΔCt。各指标重复3次取均值。
1.4 RSV对H2O2诱导的FN蛋白、COL1蛋白、NF-κB P65蛋白、P-NF-κB P65蛋白及IL-1β基因表达的影响 选取汇合度70%~80%的HTMCs,分为3组。对照组以不含H2O2及RSV的无血清培养基处理,H2O2组以300 μmol/L的H2O2处理,H2O2+RSV组同时加入300 μmol/L的H2O2及25 μmol/L 的RSV处理,以上各组均培养2 h。Western blot法检测各组FN蛋白、COL1蛋白、NF-κB P65蛋白、P-NF-κB P65蛋白的表达,方法同1.3.1。实时定量PCR法检测各组细胞IL-1β基因的表达,方法同1.3.2。
1.5 免疫荧光检测RSV对H2O2诱导的NF-κB P65核移位的影响 分组同1.4。4%多聚甲醛固定细胞10 min后,用含0.5%Triton X-100的PBS溶液透化处理5 min,PBS洗3次后,用5 g/L BSA室温封闭1 h。4℃过夜孵育一抗(NF-κB P65-抗体1∶400),PBST洗2次后,荧光二抗室温避光孵育1 h,PBST洗2次后,1 mg/L DAPI室温孵育5 min,PBS洗3次后,荧光显微镜下观察。
1.6 RSV对TGF-β2诱导的FN蛋白、COL1蛋白、NF-κB P65蛋白、P-NF-κB P65蛋白及IL-1β基因表达的影响 选取汇合度70%~80%的HTMCs,分为3组。对照组以不含TGF-β2及RSV的无血清培养基处理,TGF-β2组以5 μg/L的TGF-β2处理HTMCs,TGF-β2+RSV组为同时加入5 μg/L的TGF-β2及25 μmol/L的RSV处理,以上各组均培养12 h。Western blot法检测各组细胞FN蛋白、COL1蛋白、NF-κB P65蛋白、P-NF-κB P65蛋白的表达,方法同1.3.1。实时定量PCR法检测各组细胞IL-1β基因的表达,方法同1.3.2。
1.7 统计学方法 采用SPSS 20.0统计学软件进行数据处理。符合正态分布的计量资料用±s表示。多组间比较采用单因素方差分析(ANOVA),多样本组间两两比较先进行方差齐性检验,方差齐采用LSD-t法,方差不齐采用Dunnett T3法。P<0.05为差异有统计学意义。
Tab.1 Comparison of related protein and gene expressions between H2O2-treated groups表1H2O2不同浓度组相关蛋白和基因表达水平比较 (±s)
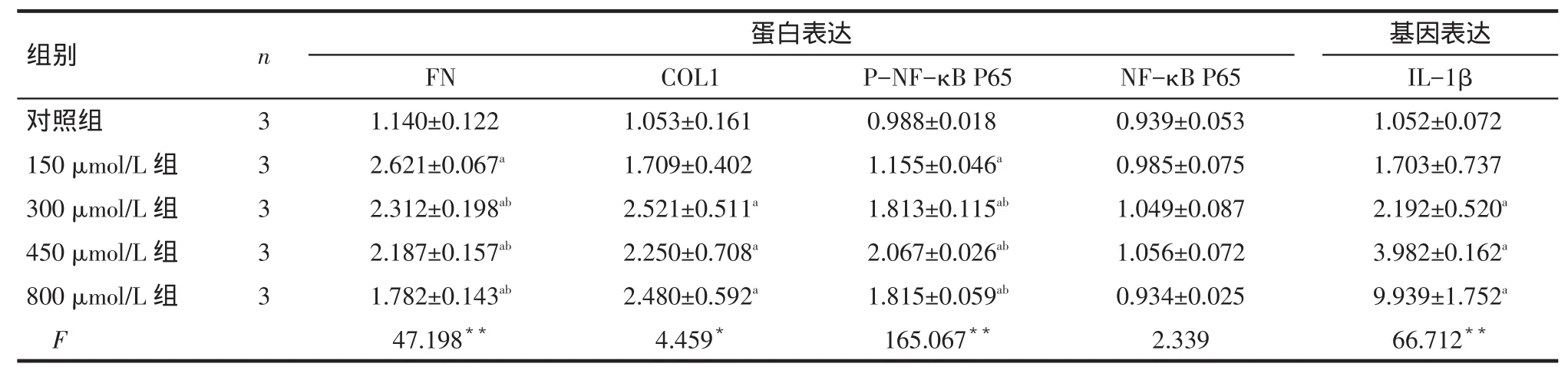
Tab.1 Comparison of related protein and gene expressions between H2O2-treated groups表1H2O2不同浓度组相关蛋白和基因表达水平比较 (±s)
*P<0.05,**P<0.01;a与对照组比较,b与150 μmol/L组比较,P<0.05
组别对照组150 μmol/L组300 μmol/L组450 μmol/L组800 μmol/L组F n 3 3 3 3 3蛋白表达FN 1.140±0.122 2.621±0.067a2.312±0.198ab2.187±0.157ab1.782±0.143ab47.198**COL1 1.053±0.161 1.709±0.402 2.521±0.511a2.250±0.708a2.480±0.592a4.459*P-NF-κB P65 0.988±0.018 1.155±0.046a1.813±0.115ab2.067±0.026ab1.815±0.059ab165.067**NF-κB P65 0.939±0.053 0.985±0.075 1.049±0.087 1.056±0.072 0.934±0.025 2.339基因表达IL-1β 1.052±0.072 1.703±0.737 2.192±0.520a3.982±0.162a9.939±1.752a66.712**
2 结果
2.1 H2O2不同浓度组相关蛋白和基因表达水平比较 与对照组比较,150、300、450、800 μmol/L组FN 和P-NF-κB P65蛋白表达水平均增高,300、450、800 μmol/L组COL1蛋白和IL-1β基因表达水平增高(P<0.05),其他指标比较差异均无统计学意义。与150 μmol/L组比较,300、450、800 μmol/L组PNF-κB P65蛋白表达水平增高,FN蛋白表达水平下降,但仍高于对照组(P<0.05)。不同浓度组NF-κB P65蛋白表达差异无统计学意义,见表1、图1。
2.2 RSV干预对H2O2诱导的HTMCs中相关指标的影响 各组NF-κB P65蛋白表达水平差异无统计学意义。H2O2组较对照组FN蛋白、COL1蛋白、PNF-κB P65蛋白及IL-1β基因表达水平均增高,而H2O2+RSV组较H2O2组上述指标均降低(P<0.05)。H2O2+RSV组较对照组仅IL-1β降低(P<0.05),其他指标差异均无统计学意义,见图2、表2。
2.3 RSV干预对TGF-β2诱导的HTMCs中相关指标的影响 各组NF-κB P65蛋白表达水平差异无统计学意义。TGF-β2组较对照组FN蛋白、COL1蛋白、P-NF-κB P65蛋白和IL-1β基因水平表达均增高(P<0.05),TGF-β2+RSV组较TGF-β2组上述指标均降低(P<0.05),见图3、表3。
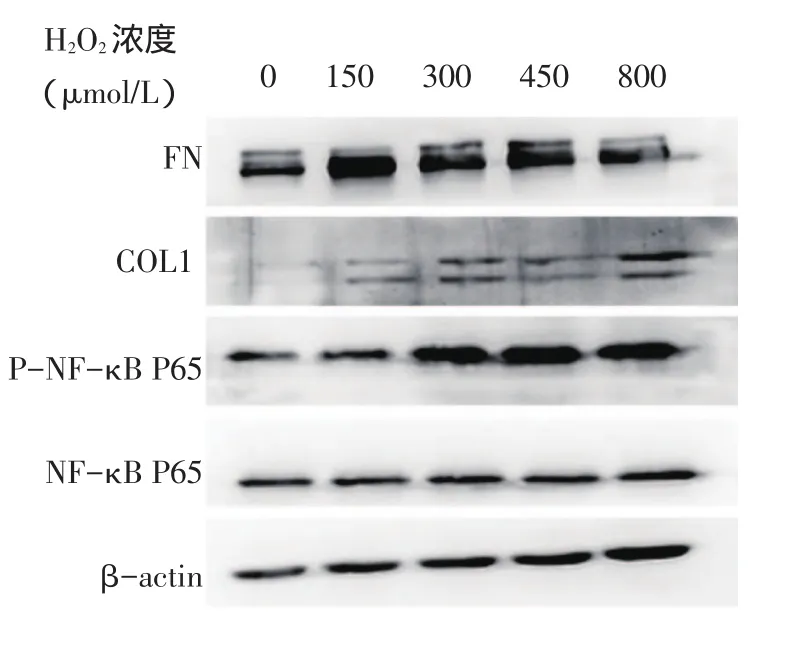
Fig.1 Comparison of related protein expressions between H2O2-treated groups图1 H2O2干预下各组相关蛋白表达水平比较
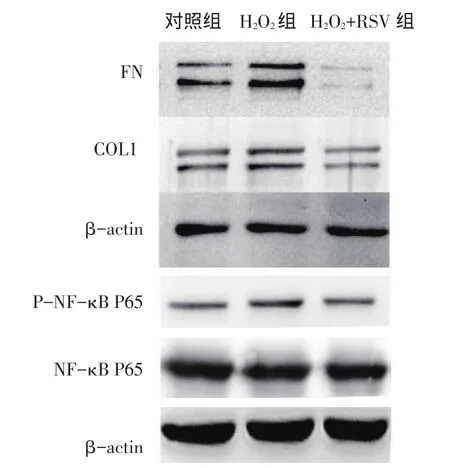
Fig.2 Comparison of H2O2-induced related protein expressions under RSV treatment图2 RSV对H2O2干预下各组相关蛋白表达水平比较
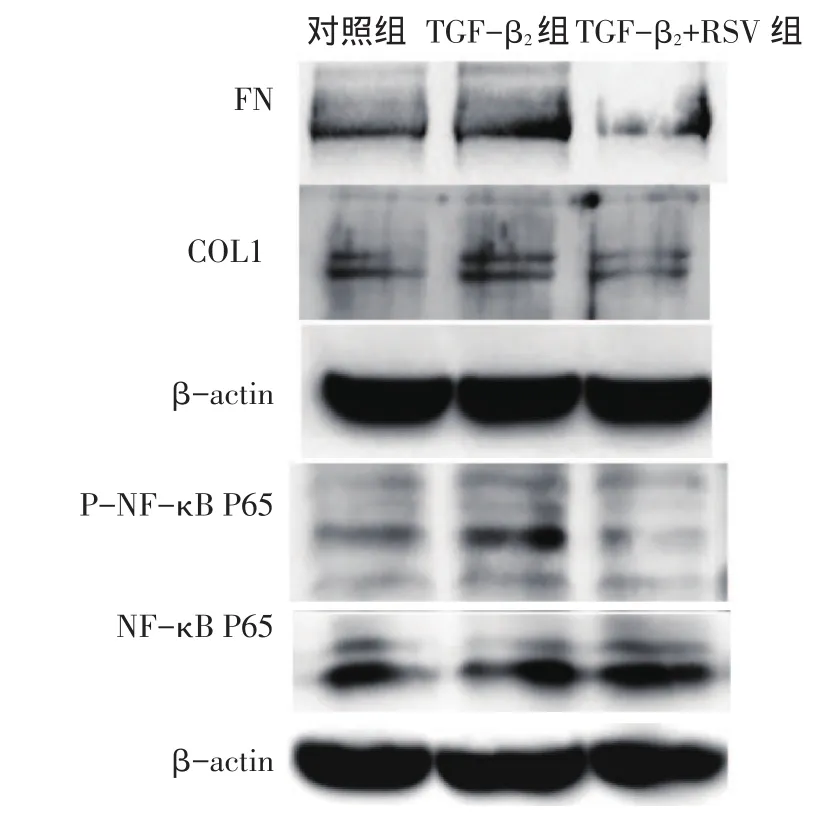
Fig.3 Comparison of TGF-β2-induced related protein expressions under RSV treatment图3 RSV对TGF-β2干预下各组相关蛋白表达水平比较
Tab.2 Comparison of H2O2-induced related protein and gene expressions under RSV treatment表2 RSV对H2O2干预下各组相关蛋白和基因表达水平比较 (±s)
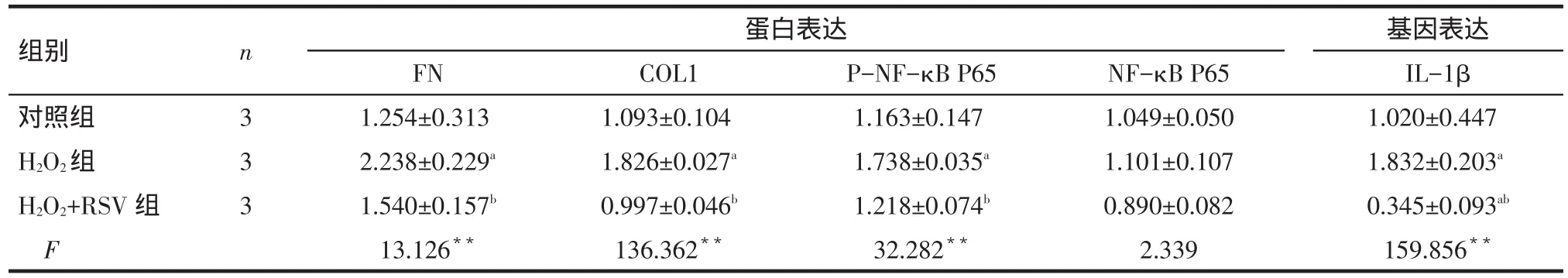
Tab.2 Comparison of H2O2-induced related protein and gene expressions under RSV treatment表2 RSV对H2O2干预下各组相关蛋白和基因表达水平比较 (±s)
**P<0.01;a与对照组比较,b与H2O组比较,P<0.05
组别对照组H2O2组H2O2+RSV组F n 3 3 3蛋白表达FN 1.254±0.313 2.238±0.229a1.540±0.157b13.126**COL1 1.093±0.104 1.826±0.027a0.997±0.046b136.362**P-NF-κB P65 1.163±0.147 1.738±0.035a1.218±0.074b32.282**NF-κB P65 1.049±0.050 1.101±0.107 0.890±0.082 2.339基因表达IL-1β 1.020±0.447 1.832±0.203a0.345±0.093ab159.856**
2.4 RSV干预对H2O2诱导的NF-κB p65蛋白核移位的影响 对照组NF-κB P65只在细胞胞质中表达;H2O2组细胞的胞质及核中均有NF-κB P65表达,且部分细胞中细胞核表达NF-κB P65较细胞质中多;H2O2+RSV组的大部分细胞的细胞质中表达NF-κB P65较细胞核中多,见图4。
3 讨论
Tab.3 Comparison of TGF-β2-induced related protein and gene expressions under RSV treatment表3 RSV对TGF-β2干预下各组相关蛋白和基因表达水平比较 (±s)
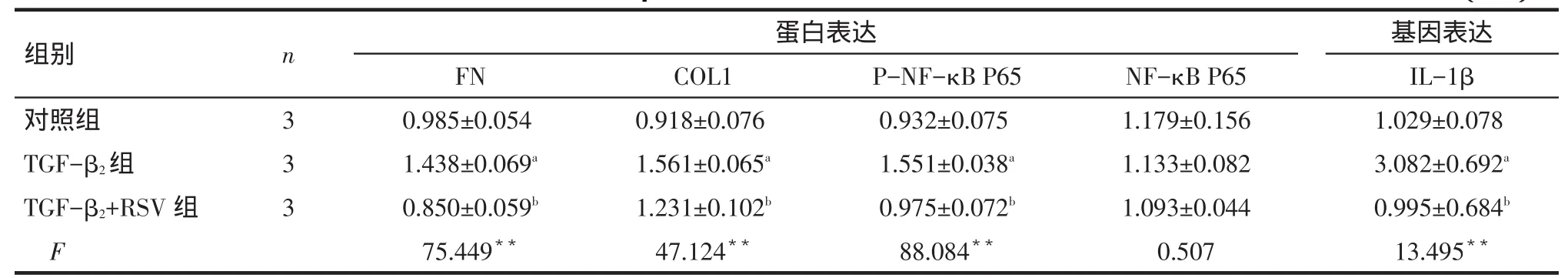
Tab.3 Comparison of TGF-β2-induced related protein and gene expressions under RSV treatment表3 RSV对TGF-β2干预下各组相关蛋白和基因表达水平比较 (±s)
**P<0.01;a与对照组比较,b与TGF-β2组比较,P<0.05
组别对照组TGF-β2组TGF-β2+RSV组F n 3 3 3蛋白表达FN 0.985±0.054 1.438±0.069a0.850±0.059b75.449**COL1 0.918±0.076 1.561±0.065a1.231±0.102b47.124**P-NF-κB P65 0.932±0.075 1.551±0.038a0.975±0.072b88.084**NF-κB P65 1.179±0.156 1.133±0.082 1.093±0.044 0.507基因表达IL-1β 1.029±0.078 3.082±0.692a0.995±0.684b13.495**
研究显示,青光眼眼压升高与TMCs的ECM异常沉积有关[2]。氧化应激是造成ECM异常积聚的重要机制之一[3]。Shen等[11]用300 μmol/L的H2O2处理HTMCs 2 h构建氧化应激损伤模型,发现ECM成分FN、COL1等表达水平升高。Li等[6]用200 μmol/L的H2O2处理猪TMCs造成慢性氧化应激后,发现NF-κB活性及炎性因子IL-1表达均升高。因此,氧化应激损伤不仅上调ECM相关蛋白表达,而且可以激活NF-κB信号通路,引发炎症反应的参与。本研究结果显示,与对照组比较,150、300、450、800 μmol/L组FN和P-NF-κB P65蛋白表达水平均增高,300、450、800 μmol/L组COL1蛋白和IL-1β基因表达水平增高,表明300 μmol/L H2O2处理HTMCs即可建立氧化应激损伤模型,从而激活NF-κB,上调炎性因子表达,造成ECM成分异常积聚。Yun等[12]用1 μmol/L的H2O2处理间充质干细胞,分别在处理15、30、60、90 min时检测发现NF-κB蛋白表达水平无明显变化,但其磷酸化水平均升高;在处理12 h 时 FN表达下降,基质金属蛋白酶(matrix metalloproteinase,MMP)-12表达升高,表明H2O2可通过激活NF-κB来上调MMP相关蛋白的表达,使FN蛋白降解。本研究结果显示,与对照组比较,150、300、450、800 μmol/L组NF-κB P65蛋白表达水平无明显变化,与150 μmol/L组比较,300、450、800 μmol/L组P-NF-κB P65蛋白表达水平增高,FN蛋白表达水平下降,与上述研究结果相近。因此,结合相关研究,笔者认为,FN蛋白在低浓度的H2O2处理下升高明显,在高浓度的H2O2处理下表达下降,可能与相关MMP蛋白表达升高,进而导致FN蛋白发生降解有关。
RSV为多酚化合物,具有抗炎、抗氧化等多种生物活性[8]。研究表明,RSV可有效抑制氧化应激状态下活性氧的增多及炎性因子的表达[9-10]。另外,RSV可通过上调去乙酰化酶(sirtuin type 1,SIRT1)表达活性,从而延缓TMCs衰老[13]。以上研究均表明RSV对TMCs的氧化应激损伤具有一定保护作用,然而有关RSV对TMCs的ECM成分的影响如何均未明确。本研究结果显示,与H2O2组相比,H2O2+RSV组FN、COL1、P-NF-κB P65的蛋白和IL-1β基因表达水平均降低,NF-κB P65核移位现象也被有效抑制,提示RSV可有效抑制H2O2诱导的NF-κB信号通路激活以及ECM相关蛋白的上调,从而发挥对TMCs的保护作用。但是,本研究还发现,H2O2+RSV组FN、COL1、P-NF-κB P65蛋白表达与对照组无明显差异,因此,RSV在一定程度上可体现出对HTMCs的保护作用,但仍需结合其他手段对青光眼进行综合治疗。
TGF-β2是存在于人眼房水中的多功能多肽,其在原发性OAG患者房水中的浓度明显高于正常人[14]。研究表明,TGF-β2可诱导TMCs的ECM成分表达增加[15-16]。TGF-β可通过激活NF-κB P65的核移位来促进FN的表达,从而影响肺纤维化过程[17]。NF-κB P65作为转录因子还可以调控FN的转录水平,从而参与上皮间质转化过程[18]。ECM成分FN、COL1异常沉积加快了心肌的纤维化,NF-κB抑制剂PDTC可通过抑制NF-κB活性,从而阻止FN、COL1的异常积聚[19]。因此,TGF-β2与NF-κB均能调控FN、COL1的表达。本研究结果显示,与对照组相比,TGF-β2组FN、COL1、P-NF-κB P65蛋白和IL-1β基因表达水平均增高,提示在HTMCs中,TGF-β2诱导FN、COL1表达增加可能与NF-κB的激活密切相关。有关肾纤维化的相关研究表明,RSV可通过上调SIRT1来抑制TGF-β信号通路,降低COL1、FN的表达[20-21]。本研究结果显示,与TGF-β2组相比,TGF-β2+RSV组FN、COL1、P-NF-κB P65蛋白和IL-1β基因表达水平均降低,证实了RSV能有效抑制TGF-β2诱导的FN、COL1表达增加及NF-κB的激活。然而,TGF-β2+RSV组NF-κB P65与TGF-β2组差异无统计学意义,表明RSV并不能完全抑制ECM的异常积聚。
综上所述,青光眼的发生、发展与氧化应激损伤、TGF-β2及NF-κB信号通路密切相关。RSV可通过抑制NF-κB的激活、降低HTMCs炎性因子及ECM蛋白的表达,从而对青光眼TMCs的应激损伤产生一定的保护作用。RSV有望成为治疗青光眼的一种潜在药物。
(图4见插页)
[1]Tham YC,Li X,Wong TY,et al.Global prevalence of glaucoma and projections of glaucoma burden through 2040:a systematic review and meta-analysis[J].Ophthalmology,2014,121(11):2081-2090.doi:10.1016/j.ophtha.2014.05.013.
[2]Vranka JA,Kelley MJ,Acott TS,et al.Extracellular matrix in the trabecular meshwork:intraocular pressure regulation and dysregulation in glaucoma[J].Exp Eye Res,2015,133:112-125.doi:10.1016/j. exer.2014.07.014.
[3]Nita M,Grzybowski A.The role of the reactive oxygen species and oxidative stress in the pathomechanisms of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults[J].Oxid Med Cell Longev,2016,2016:3164734.doi:10.1155/2016/3164734.
[4]Medina-Ortiz WE,Belmares R,Neubauer S,et al.Cellular fibronectin expression in human trabecular meshwork and induction by transforming growth Factor-β2[J].Invest Ophthalmol Vis Sci,2013,54(10):6779-6788.doi:10.1167/iovs.13-12298.
[5]Luna C,Li G,Qiu J,et al.Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress[J].Mol Vis,2009,15:2488-2497.
[6]Li G,Luna C,Liton PB,et al.Sustained stress response after oxidative stress in trabecular meshwork cells[J].Mol Vis,2007,13:2282-2288.
[7]Taurone S,Ripandelli G,Pacella E,et al.Potential regulatory molecules in the human trabecular meshwork of patients with glaucoma: immunohistochemical profile of a number of inflammatory cytokines[J].Mol Med Rep,2015,11(2):1384-1390.doi:10.3892/mmr.2014.2772.
[8]Bola C,Bartlett H,Eperjesi F.Resveratrol and eye:activity and molecular mechanisms[J].Graefes Arch Clin Exp Ophthalmol,2014,252(5):699-713.doi:10.1007/s00417-014-2604-8.
[9]Ammar DA,Hamweyah KM,Kahook MY.Antioxidants protect trabecular meshwork cells from hydrogen peroxide-induced cell death [J].Transl Vis Sci Technol,2012,1(1):4.doi:10.1167/tvst.1.1.4.
[10]Luna C,Li G,Liton PB,et al.Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells[J].Food Chem Toxicol,2009,47(1):198-204.doi:10.1016/j.fct.2008.10.029.
[11]Shen W,Han Y,Huang B,et al.MicroRNA-483-3p inhibits extracellular matrix production by targeting smad4 in human trabecular meshwork cells[J].Invest Ophthalmol Vis Sci,2015,56 (13):8419-8427.doi:10.1167/iovs.15-18036.
[12]Yun SP,Lee SJ,Oh SY,et al.Reactive oxygen species induce MMP12-dependent degradation of collagen 5 and fibronectin to promote the motility of human umbilical cord- devived mesenchymal stem cells[J].Br J Pharmacol,2014,171(13):3283-3297.doi:10.1111/bph.12681.
[13]Ren ML,Fan XJ,Yang XL,et al.SIRT1 promote GTM cell DSBs repair and resist cellular senescence[J].Journal of Sichuan University(Medical Science Edition),2014,45(4):572-577.[任朋亮,范雪娇,杨晓龙,等.SIRT1增强青光眼小梁网细胞DSBs修复能力及抗细胞衰老的研究[J].四川大学学报(医学版),2014,45(4):572-577.doi:10.13464/j.scuxbyxb.2014.04.006.
[14]Tripathi RC,Li J,Chan WF,et al.Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2[J].Exp Eye Res,1994,59(6):723-727.
[15]Wordinger RJ,Fleenor DL,Hellberg PE,et al.Effects of TGF-β2,BMP-4,and gremlin in the trabecular meshwork:Implications for glaucoma[J].Invest Ophthalmol Vis Sci,2007,48(3):1191-1200. doi:10.1167/iovs.06-0296.
[16]Webber HC,Bermudez JY,Sethi A,et al.Crosstalk between TGFβand Wntsignalingpathwaysin thehuman trabecular meshwork[J].Exp Eye Res,2016,148:97-102.doi:10.1016/j. exer.2016.04.007.
[17]Sun X,Chen E,Dong R,et al.Nuclear factor(NF)-kappaB p65 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta[J].Life Sciences,2015,122:8-14.doi:10.1016/j.lfs.2014.11.033.
[18]Stanisavljevic J,Porta-de-la-Riva M,Batlle R,et al.The p65 subunit of NF-kappaB and PARP1 assist Snail1 in activating fibronectin transcription[J].J Cell Sci,2011,124(24):4161-4171.doi:10.1242/jcs.078824.
[19]Kumar S,Seqqat R,Chigurupati S,et al.Inhibition of nuclear factor kappaB regresses cardiac hypertrophy by modulating the expression of extracellular matrix and adhesion molecules[J].Free Radic Biol Med,2011,50(1):206-215.doi:10.1016/j. freeradbiomed.2010.10.711.
[20]Xiao Z,Chen C,Meng T,et al.Resveratrol attenuates renal injury and fibrosis by inhibiting transforming growth factor-β pathway on matrix metalloproteinase 7[J].Exp Biol Med(Maywood),2016,241(2):140-146.doi:10.1177/1535370215598401.
[21]Huang XZ,Wen D,Zhang M,et al.Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-β/Smad3 pathway[J].J Cell Biochem,2014,115(5):996-1005.doi:10.1002/jcb.24748.
(2016-03-11收稿 2016-04-29修回)
(本文编辑 陆荣展)
The role and mechanism of resveratrol on trabecular meshwork cells induced by H2O2and TGF-β2
QI Yan1,2,ZHAO Xiujuan2,XU Linqi1,2,WU Xudong2△,WANG Jiantao1△
1 Tianjin Medical University Eye Hospital,School of Optometry and Ophthalmology,Eye Institute,Tianjin 300384,China;2 Deparment of Cell Biology,College of Basic Medicine,Tianjin Medical University△
Objective To investigate hydrogen peroxide(H2O2)and transforming growth factor-β2(TGF-β2)induced fibronectin(FN),collagen 1(COL1),nuclear factor(NF)-κB P65 proteins and interlukin(IL)-1β gene expression in human trabecular meshwork cells(HTMCs),and the interventional mechanism of resveratrol(RSV).Methods (1)HTMCs with 70 to 80%confluency were divided into 5 groups.The experimental groups were treated with serum-free medium and with H2O2at concentrations of 150,300,450 and 800 μmol/L.The control group was treated with 0 μmol/L H2O2.The protein levels of FN,COL1,NF-κB P65 and NF-κB P65 phosphorylation(P-NF-κB P65)were measured by Western blot assay.The expression of IL-1β gene was measured by qPCR.(2)HTMCs were divided into 3 groups.The control group was treated withserum-free medium and without H2O2and RSV.The H2O2group was treated with 300 μmol/L H2O2.The H2O2+RSV group was treated with 300 μmol/L H2O2and 25 μmol/L resveratrol(RSV).The expressions of proteins and genes mentioned above were detected in three groups.NF-κB P65 nuclear translocation was assessed by immunofluorescence technique.(3)HTMCs were divided into 3 groups.The control group was treated with serum-free medium and without TGF-β2and RSV.The TGF-β2group was treated with 5 μg/L TGF-β2.The TGF-β2+RSV group was treated with 5 μg/L TGF-β2and 25 μmol/L RSV. The expressions of proteins and genes mentioned above were detected in three groups.Results (1)Compared with control group,the protein levels of FN and P-NF-κB P65 were significantly increased in 150,300,450 and 800 μmol/L groups,the expression levels of COL1 protein and IL-1β gene were significantly increased in 300,450 and 800 μmol/L groups(P<0.05).There were no statistical significances between other indicators.(2)The expression levels of FN,COL1,P-NF-κB P65 proteins and IL-1β gene were significantly higher in H2O2group than those in control group,and which were significantly lower in H2O2+RSV group than those in H2O2group.Compared with control group,only the expression of IL-1β gene was decreased in H2O2+RSV group(P<0.05).NF-κB P65 was only expressed in cytoplasm in control group,while it was expressed in both cytoplasm and nucleus in H2O2group.Compared with H2O2group,NF-κB P65 was mainly expressed in cytoplasm.(3)Compared with control group,the expressions of FN,COL1,P-NF-κB P65 proteins and IL-1β gene were significantly increased in TGF-β2group(P<0.05).Compared with TGF-β2group,the indicators mentioned above were significantly decreased in TGF-β2+RSV group(P<0.05).Conclusion H2O2and TGF-β2can upregulate the expression of FN,COL1,P-NF-κB P65 proteins and IL-1β gene in HTMCs,which may be involved in the development and progression of glaucoma.RSV can inhibit the influence of H2O2and TGF-β2in HTMCs and exert a protective effect on glaucoma.
glaucoma;trabecular meshwork;hydrogen peroxide;transforming growth factor beta2;extracellular matrix;NF-kappa B;interleukin-1beta;protein fiber connection;collagen 1;Resveratrol
R775.2
A
10.11958/20160149
国家自然科学基金(81270994,31570774,81500170);天津市科技计划项目(10ZCKFSY08400);天津市教育委员会基金(093-201301)
1天津医科大学眼科医院,天津医科大学眼科研究所,天津医科大学眼视光学院(邮编300384);2天津医科大学基础医学院细胞生物学系
齐艳(1990),女,硕士在读,主要从事眼视光学、青光眼研究
△通讯作者 吴旭东E-mail:wuxudong@tijmu.edu.cn;汪建涛E-mail:wangjiantao65@126.com



