陈玺龙 王宏君 宋征宇 王静
摘要:目的 探究牛蒡苷元(AG)能否通过抑制高迁移率族蛋白B1(HMGB1)/Toll样受体4(TLR4)/核因子κB(NF-κB)通路的活化,从而减轻急性脑梗死大鼠神经元损伤。方法 通过大脑中动脉栓塞(MCAO)建立大鼠急性脑梗死模型后,将50只大鼠随机分为模型组、尼莫地平组(30 mg/kg)以及AG低(25 mg/kg)、中(50 mg/kg)、高(100 mg/kg)剂量组,每组10只,另取10只为假手术组(仅麻醉、游离血管,不进行插线操作)。Morris水迷宫实验评估大鼠认知功能;酶联免疫吸附试验(ELISA)检测血清肿瘤坏死因子α(TNF-α)、白细胞介素(IL)-1β、IL-6水平;HE染色与TUNEL染色观察大脑皮层病理学变化与神经元凋亡情况,Western blot检测高迁移率族蛋白B1(HMGB1)、Toll样受体4(TLR4)、核因子κB(NF-κB)、磷酸化NF-κB(p-NF-κB)蛋白表达情况。结果 与假手术组比较,模型组认知功能下降,血清TNF-ɑ、IL-1β、IL-6水平升高,神经元凋亡指数及大脑皮层HMGB1、TLR4蛋白表达和p-NF-κB/NF-κB比值升高(P<0.05),大脑皮层神经元排列紊乱,出现严重空泡化和水肿现象,细胞核固缩;与模型组比较,AG各剂量组和尼莫地平组大鼠认知功能部分恢复,血清TNF-ɑ、IL-1β、IL-6水平下降,神经元凋亡指数及大脑皮层HMGB1、TLR4蛋白表达和p-NF-κB/NF-κB比值降低(P<0.05),大脑皮层神经元损伤减轻。结论 AG可能通过抑制HMGB1/TLR4/NF-κB通路活化,减轻急性脑梗死大鼠神经元损伤。
关键词:高迁移率族蛋白B1;Toll样受体4;核因子κB;脑梗死;急性病;神经元;牛蒡苷元
中图分类号:R285.5文献标志码:ADOI:10.11958/20221432
Arctigenin alleviates neuronal damage of acute cerebral infarction in rats by inhibiting the HMGB1/TLR4/NF-κB pathway
CHEN Xilong WANG Hongjun SONG Zhengyu WANG Jing
1 Department of Neurology, 2 Department of Traditional Chinese Medicine, the First Affiliated Hospital of Hebei North University, Zhangjiakou 075000, China
Correspondence Author E-mail: dyrjn67@163.com
Abstract: Objective To explore whether arctigenin (AG) can reduce neuronal damage of acute cerebral infarction in rats by inhibiting the expression of high mobility group box 1 (HMGB1)/Toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) pathway. Methods The rat model of acute cerebral infarction was established by middle cerebral artery embolism (MCAO). Fifty model rats were randomly separated into the model group, the nimodipine group (30 mg/kg), the low AG group (25 mg/kg), the medium AG group (50 mg/kg) and the high AG group (100 mg/kg), 10 rats in each group. Another 10 rats were used as the sham operation group (only anesthesia, dissociation of blood vessel, no thrombus insertion operation). Morris water maze experiment was used to assess cognitive function in rats. The serum TNF-ɑ, IL-1β and IL-6 contents were tested by ELISA. HE staining and TUNEL staining were performed to observe the pathological changes and neuronal apoptosis of cerebral cortex, and Western blot assay was performed to measure the protein expression of HMGB1, TLR4, p-NF-κB and NF-κB. Results Compared with the sham operation group, the cognitive function was declined in the model group, serum levels of TNF-ɑ, IL-1β and IL-6 were increased, neuronal apoptosis index, cortical HMGB1 and TLR4 protein expression and p-NF-κB/NF-κB ratio were increased (P<0.05). The neuron arrangement of cerebral cortex was disordered, serious vacuolation, edema and nuclear condensation occurred. Compared with the model group, cognitive function was partially restored in the AG groups and the nimodipine group. Serum levels of TNF-ɑ, IL-1β and IL-6 decreased. Neuronal apoptosis index, cortical HMGB1, TLR4 protein expression and p-NF-κB/NF-κB ratio were decreased (P<0.05). Cortical neuron damage was reduced. (P<0.05). Conclusion AG may inhibit the HMGB1/TLR4/NF-κB pathway to reduce neuronal damage in rats with acute cerebral infarction.
Key words: high mobility group box 1; Toll-like receptor 4; nuclear factor κB; cerebral infarction; acute disease; neuron; Arctigenin
急性脑梗死多发于中老年人,发病率较高,致死、致残率也较高[1]。其发病机制为脑组织血氧不足,发生氧化应激与炎症反应,从而诱导氧自由基生成以及神经元凋亡、机体酸中毒等病理过程的发生与发展[2]。高迁移率族蛋白B1(HMGB1)是一种非组蛋白结合蛋白,是炎症反应晚期的标志物[3];Toll样受体4(TLR4)对应激炎症反应的调节至关重要[4];HMGB1与TLR4结合后参与炎症反应、免疫应激以及细胞凋亡等生理病理过程[5]。研究表明,HMGB1通过调控炎症反应,激活TLR4,从而诱导核因子κB(NF-κB)活化,介导一系列炎性因子,包括白细胞介素(IL)-1β、肿瘤坏死因子ɑ(TNF-ɑ)、IL-6等的分泌,进一步加剧炎症反应,加重组织损伤[6]。因此,调控HMGB1/TLR4/NF-κB通路的表达活性,可改善炎症反应,在急性脑梗死的治疗中具有关键作用。牛蒡苷元(AG)是牛蒡的主要生物活性成分,具有抗炎、抗氧化、抗肿瘤以及抗凋亡等多种作用[7-8]。研究表明,AG能够通过抑制HMGB1/TLR4/NF-κB通路活化,从而抑制小胶质细胞激活以及神经炎症反应,发挥抗抑郁作用[9]。然而,AG对急性脑梗死的影响及作用机制研究鲜见。本研究通过建立急性脑梗死大鼠模型,探究AG能否通过抑制HMGB1/TLR4/NF-κB通路的激活,改善急性脑梗死大鼠神经元损伤,以期为AG的应用和急性脑梗死的防治提供参考。
1 材料与方法
1.1 实验动物
6周龄SPF级雄性SD大鼠60只,体质量200~220 g,购自河北省实验动物中心,动物生产许可证号:SCXK(冀)2018-004。实验前在温度为22~25 ℃、湿度40%~50%、光照时间12 h的环境中适应性饲养1周,实验动物自由饮食和水。实验过程严格按照实验动物管理条例进行。
1.2 主要仪器与试剂
AG(ZSU-C-002,湖州展舒生物科技有限公司);尼莫地平(A14202005165,国药准字H14022821,亚宝药业集团股份有限公司);TNF-ɑ、IL-1β、IL-6酶联免疫吸附试验(ELISA)试剂盒、二辛可宁酸(BCA)蛋白浓度测定试剂盒、TUNEL染色试剂盒(南京建成生物工程研究所);苏木精-伊红(HE)染色试剂盒、高效RIPA裂解液(北京索莱宝生物技术有限公司);一抗HMGB1、TLR4、p-NF-κB、NF-κB、β-肌动蛋白(β-actin)和二抗羊抗兔IgG(英国Abcam公司)。Viewer小动物行为轨迹记录分析系统(德国Biobserve公司);DM3000 LED光学显微镜(德国leica公司);GenoSens 2100凝胶成像系统(上海Clinx勤翔科学仪器有限公司)。
1.3 研究方法
1.3.1 模型建立与分组给药
参照文献[10],通过大脑中动脉栓塞(MCAO)建立大鼠急性脑梗死模型:腹腔注射戊巴比妥钠(40 mg/kg)麻醉大鼠,分离左侧的颈外动脉、颈内动脉、颈总动脉,结扎颈外动脉与颈总动脉近心端,颈总动脉远心端夹闭后剪口,将线栓插入颈内动脉,进线约15 mm,感受到阻力停止进线,堵塞大脑动脉,造成急性脑梗死。将造模成功的50只大鼠随机分为模型组,AG低、中、高剂量组和尼莫地平组,每组10只。另取10只为假手术组,仅麻醉、游离血管,不进行插线操作。造模第2天开始灌胃给药,尼莫地平组给药30 mg/kg[11],AG低、中、高剂量组分别给药25、50及100 mg/kg[12],每日1次,连续干预1周。假手术组、模型组给予等体积生理盐水灌胃。
1.3.2 大鼠认知功能评价
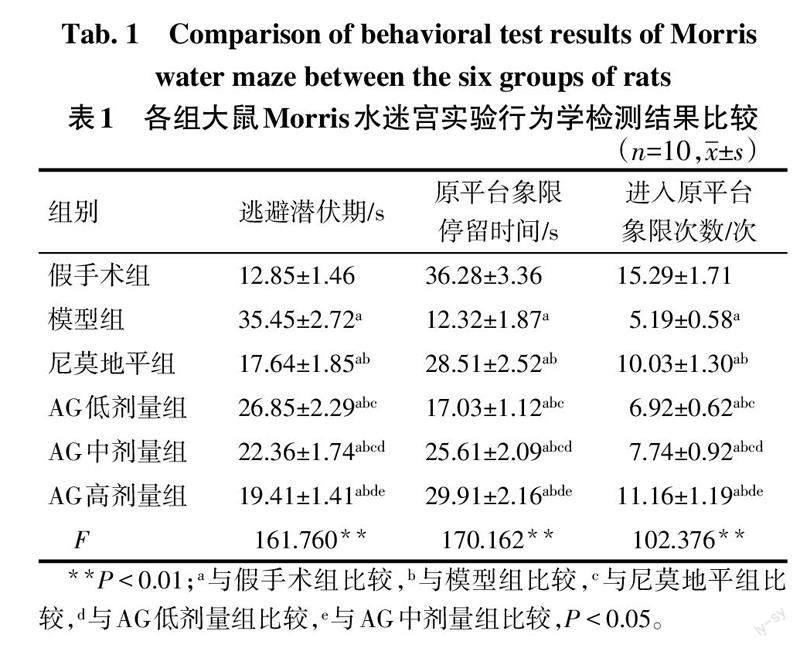
参照文献[12],采用Morris水迷宫实验评估各组大鼠认知功能:在圆形水池中设置4个象限,选取其中一个作为平台象限,记录各组大鼠找到平台的平均时间作为逃避潜伏期;5 d后撤除平台,让各组大鼠自由游泳90 s,记录大鼠在原平台象限的停留时间以及进入原平台象限次数。
1.3.3 ELISA检测血清中TNF-ɑ、IL-1β、IL-6水平
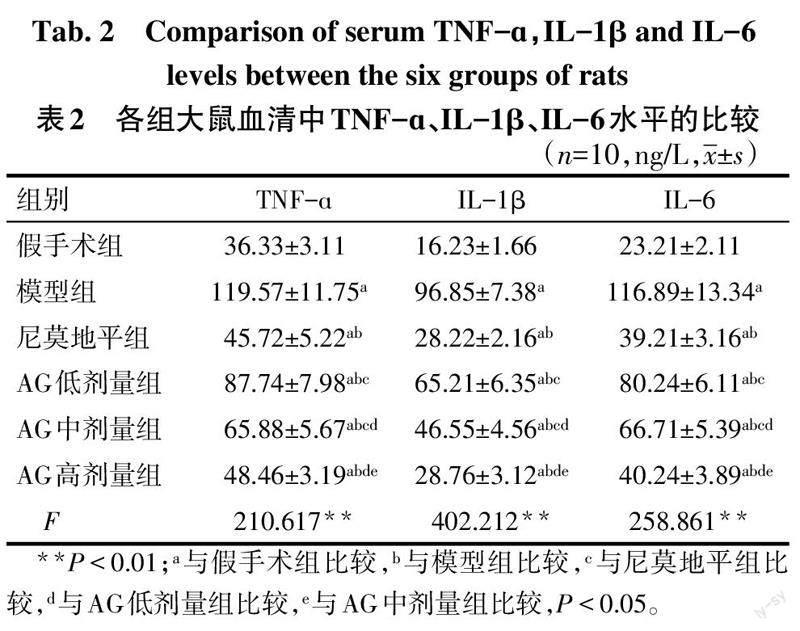
Morris水迷宫实验结束后,麻醉大鼠,腹主动脉取血2 mL,4 ℃下3 000 r/min离心10 min取血清,参照试剂盒说明书,检测TNF-ɑ、IL-1β、IL-6水平。
1.3.4 HE染色观察大脑皮层病理变化
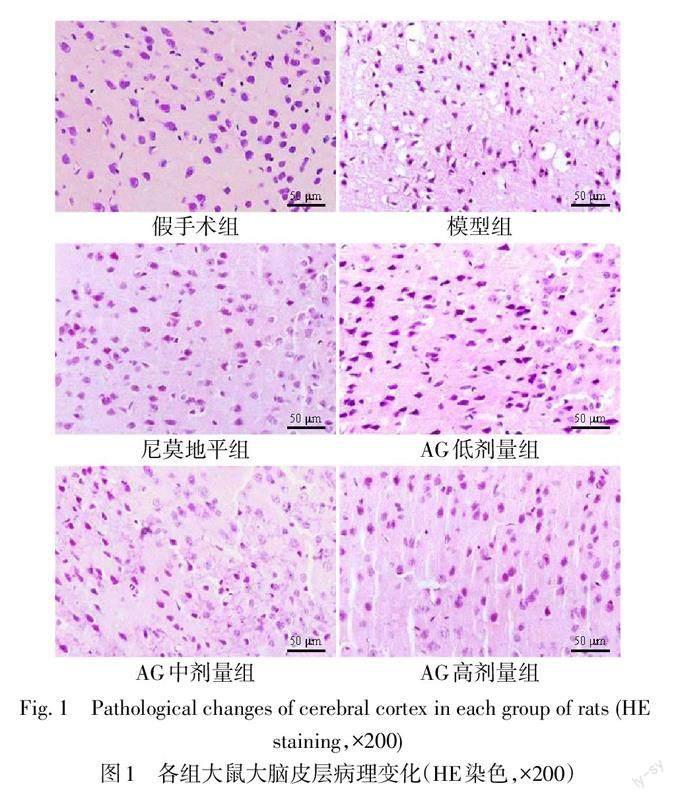
每组随机取5只大鼠,断头法处死后,迅速剥离脑组织,分离大脑皮层组织,加入4%多聚甲醛固定,常规梯度乙醇脱水,石蜡包埋、切片,备用。常规梯度乙醇复水,采用HE染色试剂盒染色,光镜下观察各组大鼠大脑皮层病理学变化。
1.3.5 TUNEL法观察大脑皮层神经元凋亡情况
取脑组织石蜡切片(厚度5 μm),常规梯度乙醇复水后加入多聚甲醛浸泡30 min,柠檬酸溶液浸泡20 min,磷酸盐缓冲液(PBS)清洗后加入TUNEL染色液进行染色,苏木精复染,梯度乙醇脱水,封片。光镜下观察各组大鼠大脑皮层神经元凋亡情况,细胞核呈棕褐色提示为凋亡神经元,凋亡指数=凋亡神经元数量/细胞总数×100%。
1.3.6 Western blot检测大脑皮层HMGB1、TLR4、NF-κB、磷酸化NF-κB(p-NF-κB)蛋白相对表达水平
每组剩余5只大鼠处死后取出完整大脑并分离皮层组织,研磨后加入高效RIPA裂解液制备匀浆,采用BCA法测定总蛋白浓度。取50 μg蛋白上样进行电泳、转膜、5%脱脂奶粉封闭,TBST清洗,加入HMGB1(1∶1 000)、TLR4(1∶1 000)、p-NF-κB(1∶500)、NF-κB(1∶1 000)一抗,孵育过夜,加入羊抗兔IgG二抗(1∶2 000),37 ℃孵育2 h,采用电化学发光(ECL)试剂盒进行显色,凝胶成像系统拍照并分析灰度值,以β-actin为内参,计算HMGB1、TLR4蛋白相对表达量和p-NF-κB/NF-κB比值。
1.4 统计学方法
采用GraphPad Prism 8.0软件进行数据分析。符合正态分布的计量数据用x±s表示,多组间比较用单因素方差分析,组间多重比较用SNK-q法。P<0.05为差异有统计学意义。
2 结果
2.1 各组大鼠认知功能比较
与假手术组比较,模型组大鼠认知功能下降(P<0.05);与模型组比较,尼莫地平组与AG低、中及高剂量组大鼠认知功能增加(P<0.05),且AG各剂量组随着剂量升高,大鼠认知功能依次增加(P<0.05)。见表1。
2.2 各组大鼠血清TNF-ɑ、IL-1β、IL-6水平比较
与假手术组比较,模型组TNF-ɑ、IL-1β、IL-6水平升高(P<0.05);与模型组比较,尼莫地平组与AG低、中及高剂量组TNF-ɑ、IL-1β、IL-6水平降低(P<0.05),且AG各剂量组间差异均有统计学意义(P<0.05);与尼莫地平组比较,AG低、中剂量组大鼠血清中TNF-ɑ、IL-1β、IL-6水平升高(P<0.05)。见表2。
2.3 各组大鼠大脑皮层病理变化比较
假手术组皮层神经元排列紧密,细胞结构完整且核清晰,未见神经元病变;模型组大鼠大脑皮层神经元排列紊乱,出现严重空泡化和水肿现象,细胞核固缩;AG各剂量组随着剂量升高,神经元空泡化和水肿现象逐渐减轻,细胞核固缩减少,细胞排列较整齐;其中AG高剂量组与尼莫地平组神经元病变程度一致,细胞结构趋于正常。见图1。
2.4 各组大鼠脑皮层神经元凋亡情况比较
假手术组、模型组、尼莫地平组、AG低剂量组、AG中剂量组、AG高剂量组脑皮层神经元凋亡指数分别为(6.25±0.65)%、(75.21±6.50)%、(16.33±1.28)%、(56.02±4.74)%、(35.25±3.35)%、(19.26±1.58)%,差异有统计学意义(F=259.473,P<0.05)。假手术组脑皮层组织中仅看到极少数凋亡神经元;与假手术组比较,模型组凋亡神经元数量增加,凋亡指数升高(P<0.05);与模型组比较,AG低、中及高剂量组凋亡指数依次降低,尼莫地平组大鼠神经元凋亡指数亦降低(P<0.05)。见图2。
2.5 各组大鼠大脑皮层HMGB1、TLR4、NF-κB和p-NF-κB蛋白表达比较
与假手术组比,模型组HMGB1、TLR4蛋白表达和p-NF-κB/NF-κB比值升高(P<0.05);与模型组比较,AG低、中及高剂量组HMGB1、TLR4蛋白表达和p-NF-κB/NF-κB比值依次降低,尼莫地平组HMGB1、TLR4蛋白表达和p-NF-κB降低(P<0.05);与尼莫地平组比较,AG高剂量组上述指标变化差异无统计学意义(P>0.05)。见图3、表3。
3 讨论
急性脑梗死因其发病急、病死率高,且发病后脑组织损伤不可逆的特点,严重威胁人们的生命安全[13]。急性脑梗死主要是由于脑血氧不足,引发炎症反应,诱导大量活性氧释放,导致神经损伤[14]。因此,改善炎症反应是治疗急性脑梗死的关键。
AG作为牛蒡中最丰富、生物活性最强的物质,常用于炎症的治疗[15]。研究发现,AG能够通过抑制HMGB1/TLR4和TNF-α/TNFR1介导的NF-κB激活,减弱小胶质细胞激活和神经炎症[16]。本研究结果亦显示,低、中、高剂量AG干预后,急性脑梗死大鼠血清中炎性因子水平下降,神经功能损伤、神经元空泡化与水肿现象均减轻,神经元凋亡减少,且高剂量AG优于低、中剂量AG,提示AG能够有效改善急性脑梗死大鼠体内炎症反应,减轻神经元损伤。尼莫地平在临床上常用作脑血管疾病以及神经系统疾病的治疗药物。本研究将其作为AG的阳性药物,结果显示,尼莫地平可显着抑制急性脑梗死大鼠体内炎症反应,减轻神经元损伤,且高剂量AG对急性脑梗死大鼠的保护作用与尼莫地平一致,再次证实AG对于急性脑梗死的治疗有效。
急性脑梗死是一系列炎症反应引起的神经元损伤,最终导致神经元坏死、凋亡[17-18]。HMGB1/TLR4/NF-κB通路是介导炎症反应的关键信号通路[19]。研究表明,HMGB1作为晚期炎性因子,参与急性脑梗死造成的神经元损伤,导致脑梗死面积增加,炎症反应加重[20]。缺血性脑损伤可引发HMGB1活化,与其受体TLR4结合,诱导下游促炎因子NF-κB表达上调,继而引发炎性因子IL-6、TNF-α水平升高,炎症反应加剧,脑损伤加重[21]。已有研究证实,抑制HMGB1/TLR4/NF-κB通路活化能够有效改善炎症反应,减轻脑损伤[22-23]。本研究结果显示,低、中、高剂量AG干预后的急性脑梗死大鼠大脑皮层组织中HMGB1、TLR4蛋白表达和p-NF-κB/NF-κB比值逐渐下降,提示AG能够通过下调HMGB1、TLR4和NF-κB磷酸化蛋白表达,抑制HMGB1/TLR4/NF-κB通路活化,改善由于脑供血不足引起的炎症反应,减少神经元凋亡。
综上所述,AG可能通过抑制HMGB1/TLR4/NF-κB通路,减轻急性脑梗死大鼠神经元损伤。本研究为急性脑梗死的治疗提供了一种候选药物,有助于治疗急性脑梗死新药的开发。
参考文献
[1] WEN H,LV M. Correlation analysis between serum procalcitonin and infarct volume in young patients with acute cerebral infarction[J]. Neurol Sci,2021,42(8):3189-3196. doi:10.1007/s10072-020-04856-x.
[2] 闫振文,郑眉光,李梅. 益气活血通络方对急性脑梗死大鼠炎症因子、氧化应激指标及神经细胞凋亡的影响[J]. 中国中医急症,2020,29(6):957-961. YAN Z W,ZHENG M G,LI M. Effects of Yiqi Huoxue Tongluo Formula on inflammatory factors,oxidative stress indexes and apoptosis of nerve cells in rats with acute cerebral infarction[J]. Chinese Journal of Traditional Chinese Medicine Emergency,2020,29(6):957-961. doi:10.3969/j.issn.1004-745X.2020.06005.
[3] XUE J,SUAREZ J S,MINAAI M,et al. HMGB1 as a therapeutic target in disease[J]. J Cell Physiol,2021,236(5):3406-3419. doi:10.1002/jcp.30125.
[4] KARIMY J K,REEVES B C,KAHLE K T. Targeting TLR4-dependent inflammation in post-hemorrhagic brain injury[J]. Expert Opin Ther Targets,2020,24(6):525-533. doi:10.1080/14728222.2020.1752182.
[5] LU Y N,ZHAO X D,XU X,et al. Arctigenin exhibits hepatoprotective activity in Toxoplasma gondii-infected host through HMGB1/TLR4/NF-κB pathway[J]. Int Immunopharmacol,2020,84:106539. doi:10.1016/j.intimp.2020.106539.
[6] SUN Z,NYANZU M,YANG S,et al. VX765 attenuates pyroptosis and HMGB1/TLR4/NF-κB pathways to improve functional outcomes in TBI mice[J]. Oxid Med Cell Longev,2020,2020:7879629. doi:10.1155/2020/7879629.
[7] QIAO S,LV C,TAO Y,et al. Arctigenin disrupts NLRP3 inflammasome assembly in colonic macrophages via downregulating fatty acid oxidation to prevent colitis-associated cancer[J]. Cancer Lett,2020,491:162-179. doi:10.1016/j.canlet.2020.08.033.
[8] LI H,ZHANG X,XIANG C,et al. Identification of phosphodiesterase-4 as the therapeutic target of arctigenin in alleviating psoriatic skin inflammation[J]. J Adv Res,2021,33:241-251. doi:10.1016/j.jare.2021.02.006.
[9] XANG X,PIAO H N,AOSAI F,et al. Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways[J]. Brit J Pharmacol,2020,177(22):5224-5245. doi:10.1111/bph.15261.
[10] XU Y,ZHANG G,KANG Z,et al. Cornin increases angio-genesis and improves functional recovery after stroke via the Ang1/Tie2 axis and the Wnt/β-catenin pathway[J]. Arch Pharm Res,2016,39(1):133-142. doi:10.1007/s12272-015-0652-1.
[11] 张立波,姚袁媛,王清勇,等. 半枝莲总黄酮调控RIP1/RIP3通路减轻急性脑梗死缺血再灌注大鼠大脑皮层神经元的损伤[J]. 中医学报,2020,35(7):1476-1484. ZHANG L B,YAO Y Y,WANG Q Y,et al. Regulation of RIP1/RIP3 pathway by total flavonoids from Schistoderma chinensis alleviates the damage of cerebral cortical neurons in rats with acute cerebral infarction ischemia reperfusion[J]. Journal of Traditional Chinese Medicine,2020,35(7):1476-1484. doi:10.16368/j.issn.1674-8999.2020.07.331.
[12] 郑建君,杨霄敏,毛玲群. 基于TGF-β1/Smad3信号通路探讨金合欢素对急性脑梗死大鼠神经功能的保护作用[J]. 中国药师,2020,23(7):1350-1354. ZHENG J J,YANG X M,MAO L Q. Protective effect of acacia on neural function of rats with acute cerebral infarction based on TGF-β1/Smad3 signal pathway[J]. Chinese Pharmacist,2020,23(7):1350-1354.
[13] JIANG Y,REN C,ALIMUJIANG A,et al. The difference in red blood cell distribution width from before to after thrombolysis as a prognostic factor in acute ischemic stroke patients: a 2-year follow-up[J]. Front Neurol,2022,13:1011946. doi:10.3389/fneur.2022.1011946.
[14] AMIN N,DU X,CHEN S,et al. Therapeutic impact of thymoquninone to alleviate ischemic brain injury via Nrf2/HO-1 pathway[J]. Expert Opin Ther Targets,2021,25(7):597-612. doi:10.1080/14728222.2021.1952986.
[15] WU D,JIN L,HUANG X,et al. Arctigenin:pharmacology,total synthesis,and progress in structure modification[J]. J Enzyme Inhib Med Chem,2022,37(1):2452-2477. doi:10.1080/14756366.2022.2115035.
[16] XU X,ZENG X Y,CUI Y X,et al. Antidepressive effect of arctiin by attenuating neuro inflammation via HMGB1/TLR4- and TNF-α/TNFR1-mediated NF-κB activation[J]. ACS Chem Neurosci,2020,11(15):2214-2230. doi:10.1021/acschemneuro.0c00120.
[17] MAIDA C D,NORRITO R L,DAIDONE M,et al. Neuroinflammatory mechanisms in ischemic stroke:focus on cardioembolic stroke,background,and therapeutic approaches[J]. Int J Mol Sci,2020,21(18):6454. doi:10.3390/ijms21186454.
[18] WANG Y,JIN H,WANG Y,et al. Sult2b1 deficiency exacerbates ischemic stroke by promoting pro-inflammatory macrophage polarization in mice[J]. Theranostics,2021,11(20):10074-10090. doi:10.7150/thno.61646.
[19] WANG Q,ZHAO H,GAO Y,et al. Uric acid inhibits HMGB1-TLR4-NF-κB signaling to alleviate oxygen-glucose deprivation/reoxygenation injury of microglia[J]. Biochem Biophys Res Commun,2021,540:22-28. doi:10.1016/j.bbrc.2020.12.097.
[20] 高元杰,钟纯正,欧诒丹. 脑梗死合并肺部感染患者TNF-α、HMGBl、 TLR4-NF-κB信号通路改变研究[J]. 中华医院感染学杂志,2020,30(7):1003-1006. GAO Y J,ZHONG C Z,OU Y D. Changes of TNF-α,HMGBl,TLR4-NF-κB signaling pathways in patients with cerebral infarction complicated with pulmonary infection[J]. Chinese Journal of Nosocomiology,2020,30(7):1003-1006. doi:10.11816/cn.ni.2020-191297.
[21] TAVERNELLI L E,MOTTA M C M,GON?ALVES C S,et al. Overexpression of Trypanosoma cruzi High Mobility Group B protein (TcHMGB) alters the nuclear structure,impairs cytokinesis and reduces the parasite infectivity[J]. Sci Rep,2019,9(1):192. doi:10.1038/s41598-018-36718-0.
[22] CHEN X,WU S,CHEN C,et al. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury[J]. J Neuroinflammation,2017,14(1):143. doi:10.1186/s12974-017-0917-3.
[23] ZHAI Y,ZHU Y,LIU J,et al. Dexmedetomidine post-conditioning alleviates cerebral ischemia-reperfusion injury in rats by inhibiting high mobility group protein B1 group (HMGB1)/toll-like receptor 4 (TLR4)/nuclear factor kappa B (NF-κB) signaling pathway[J]. Med Sci Monit,2020,26:e918617. doi:10.12659/MSM.918617.
(2022-09-13收稿 2023-01-05修回)
(本文编辑 陆荣展)



