潘娟 王琪 田艳妮
摘要 目的:甘草甜素(Gly)调节高迁移率族蛋白(HMGB1)/晚期糖基化终产物受体(RAGE)信号通路对高糖诱导的心肌细胞凋亡和自噬的影响。方法:体外培养大鼠心肌细胞H9c2,将其分为7组:对照组、高糖组、高糖+Gly组、高糖+pcDNA3.1空载体组、高糖+pcDNA3.1-HMGB1组、高糖+Gly+pcDNA3.1空载体组、高糖+Gly+pcDNA3.1-HMGB1组。细胞计数试剂盒-8(CCK-8)法检测细胞活力;V-异硫氰酸荧光素(Annexin V-FITC)/碘化丙啶(PI)法检测细胞凋亡率;透射电镜观察细胞自噬;蛋白免疫印迹法(Western Blot)检测B细胞淋巴瘤/白血病基因-2相关X蛋白基因(Bax)、微管相关蛋白1轻链3(LC3)和Beclin-1凋亡、自噬相关蛋白表达;酶联免疫吸附试验(ELISA)检测白介素-1β(IL-1β)、肿瘤坏死因子α(TNF-α)水平;2,7-二氯荧光素二乙酸酯(DCFH-DA)荧光探针法检测活性氧(ROS)水平;实时荧光定量聚合酶链式反应(qRT-PCR)检测HMGB1、RAGE mRNA表达水平。结果:与对照组比较,高糖组H9c2细胞活力下降,细胞凋亡率、自噬小体数量及Bax、LC3、Beclin-1蛋白表达增加,IL-1β、TNF-α、ROS水平及HMGB1、RAGE mRNA表达增加,差异均有统计学意义(P<0.05);与高糖组比较,高糖+Gly组H9c2细胞活力升高,细胞凋亡率、自噬小体数量及Bax、LC3、Beclin-1蛋白表达减少,IL-1β、TNF-α、ROS水平及HMGB1、RAGE mRNA表达减少,差异均有统计学意义(P<0.05);与高糖+Gly组比较,高糖+Gly+pcDNA3.1-HMGB1组H9c2细胞活力下降,细胞凋亡率、自噬小体数量及Bax、LC3、Beclin-1蛋白表达增加,IL-1β、TNF-α、ROS水平及HMGB1、RAGE mRNA表达增加,差异均有统计学意义(P<0.05)。结论:Gly能够减轻高糖诱导的H9c2细胞炎症和氧化应激反应,抑制其凋亡和自噬,可能与抑制HMGB1/RAGE信号通路有关。
关键词 甘草甜素;高迁移率族蛋白;晚期糖基化终产物受体;高糖;心肌细胞;细胞凋亡;细胞自噬;实验研究
doi:10.12102/j.issn.1672-1349.2024.11.007
Effects of Glycyrrhizin Modulating HMGB1/RAGE Signaling Pathway on Apoptosis and Autophagy of Cardiomyocytes Induced by High Glucose
PAN Juan, WANG Qi, TIAN Yanni
Xian Yang Central Hospital, Xianyang 712000, Shaanxi, China
Corresponding Author TIAN Yanni, E-mail: tianyn0516@163.com
Abstract Objective:To investigate the effect of glycyrrhizin(Gly) on apoptosis and autophagy induced by high glucose in cardiomyocytes by regulating the high mobility group box protein(HMGB1)/receptor for advanced glycation end products(RAGE) signaling pathway.Methods:Rat cardiomyocytes H9c2 were cultured in vitro and divided into control(Ctrl) group,high glucose group,high glucose+Gly group,high glucose+pcDNA3.1 empty vector group,high-glucose+pcDNA3.1-HMGB1 group,high glucose+Gly+pcDNA3.1 empty vector group,and high glucose+Gly+pcDNA3.1-HMGB1 group.Cell viability was detected by cell counfing kit-8(CCK-8) assay.Apoptosis rate was detected by Annexin V-FITC/propyl iodide(PI) method.Autophagy was observed by transmission electron microscopy.The expression of B-cell lymphoma/leukemia gene-2-related X protein gene(Bax),microtubule-related protein 1 light chain 3(LC3) and Beclin-1 apoptosis and autophagy-related protein were detected by Western Blot.The levels of interleukin-1β(IL-1β) and tumor necrosis factor α(TNF-α) were detected by enzyme-linked immunosorbent assay(ELISA).2,7-dichlorofluorescein diacetate(DCFH-DA) fluorescence probe method was performed to detect reactive oxygen species(ROS).Real-time fluorescence quantitative polymerase chain reaction(qRT-PCR) was performed to detect the mRNA expression levels of HMGB1 and RAGE.Results:Compared with Ctrl group,H9c2 cell viability inhigh glucose group decreased,apoptosis rate,autophagosome number and protein expression of Bax,LC3 and Beclin-1 increased,IL-1β,TNF-α,ROS levels and mRNA expression of HMGB1 and RAGE increased,with statistical significance(P<0.05).Compared with the high glucose group,the H9c2 cell viability was increased in the high glucose+Gly group,the apoptosis rate,the number of autophagosomes and the expression of Bax,LC3 and Beclin-1 protein decreased,and the levels of IL-1β,TNF-α and ROS and the mRNA expressions of HMGB1 and RAGE decreased,with statistical significance(P<0.05).Compared with the high glucose+Gly group,the viability of H9c2 cells in the high glucose+Gly+pcDNA3.1-HMGB1 group decreased,the apoptosis rate,the number of autophagosomes and the expression of Bax,LC3 and Beclin-1 protein increased,the levels of IL-1β,TNF-α and ROS and the expression of HMGB1 and RAGE mRNA increased.The differences were statistically significant(P<0.05).Conclusion:Gly can reduce the inflammation and oxidative stress response of H9c2 cells induced by high glucose,inhibit their apoptosis and autophagy,which may be related to the inhibition of HMGB1/RAGE signaling pathway.
Keywords glycyrrhizin; high mobility group box protein; receptor for advanced glycation end products;high glucose; cardiomyocytes; apoptosis; autophagy; experimental study
糖尿病性心肌病(diabetic cardiomyopathy,
通讯作者 田艳妮,E-mail:tianyn0516@163.com
引用信息 潘娟,王琪,田艳妮.甘草甜素调节HMGB1/RAGE信号通路对高糖诱导的心肌细胞凋亡和自噬的影响[J].中西医结合心脑血管病杂志,2024,22(11):1961-1966.
DCM)是一种以心脏结构和功能的异常改变为特征的糖尿病并发症,也是引发糖尿病病人死亡的常见原因之一[1]。研究显示,炎症和氧化应激反应在DCM的发生进展中发挥着关键性作用,过度累积的炎性因子和活性氧(reactive oxygen species,ROS)可诱导心脏收缩功能受损,促进心肌细胞凋亡和自噬[2-3]。因此,抑制炎症和氧化应激反应是防治DCM的重要方向。
甘草甜素(glycyrrhizin,Gly)是从中药甘草的根中分离得到的一种三萜类化合物,具有抗炎、抗氧化、抗病毒、免疫调节、抗溃疡等广泛的药理生物活性[4]。有研究显示,Gly对心脏损伤有一定的保护作用[5]。但关于Gly对DCM的作用及潜在机制目前仍不清楚。高迁移率族蛋白1(high mobility group box-1 protein,HMGB1)/晚期糖基化终产物受体(receptor of advanced glycation end-product,RAGE)作为在诱导炎症和氧化应激反应中发挥重要作用的信号通路已被广泛研究,且研究显示HMGB1/ RAGE参与糖尿病及相关并发症的发生进展[6-7]。此外,有研究报道称Gly可通过抑制HMGB1/RAGE信号通路而改善2,4-二硝基氯苯诱导的小鼠特应性皮炎症状或减轻脑静脉窦阻塞再通引发的脑损伤[8-9]。本研究利用高糖诱导大鼠心肌细胞H9c2损伤,观察Gly对H9c2细胞凋亡和自噬的影响,同时分析HMGB1/RAGE信号通路在其中的作用,初步揭示Gly在DCM疾病中的调控机制。
1 材料与方法
1.1 材料
ATCC来源大鼠H9c2细胞购自上海雅吉生物科技有限公司;Gly购自上海恒斐生物科技有限公司;pcDNA3.1空载体及pcDNA3.1-HMGB1购自上海吉玛制药公司;Lipofectamine-2000购自上海玉博生物科技有限公司;细胞计数(CCK-8)试剂盒、V-异硫氰酸荧光素(Annexin V-FITC)/碘化丙啶(propidium iodide,PI)试剂盒、ROS
2,7-二氯荧光素二乙酸酯(DCFH-DA)荧光探针试剂盒、RIPA裂解液、二喹啉甲酸(BCA)试剂盒购自上海碧云天生物公司;大鼠白介素-1β(interleukin-1β,IL-1β)、肿瘤坏死因子α(tumor necrosis factor α,TNF-α)酶联免疫吸附试验(ELISA)试剂盒购自上海酶研生物科技有限公司;Trizol购自上海联硕生物科技有限公司;HMGB1、RAGE引物由北京全式金生物公司设计合成;兔源一抗anti-B细胞淋巴瘤/白血病基因-2相关X蛋白基因(B-cell lymphoma/leukemia gene-2 associated X Protein,Bax)、anti-微管相关蛋白1轻链3(microtu-bule associated protein 1 light chain 3,LC3)、anti-Beclin-1和山羊抗兔二抗购自Abcam公司。
1.2 细胞处理及分组
H9c2细胞采用低糖(5.5 mmol/L)杜尔伯科改良伊格尔(DMEM)培养基,于37 ℃、5%CO2培养箱中常规培养。待细胞密度约80%时收集,以1×106个/孔接种至6孔板内,待细胞密度约80%时利用Lipofectamine-2000分别将pcDNA3.1空载体、pcDNA3.1-HMGB1转染至H9c2细胞中,同时设置未转染的H9c2细胞,48 h后经实时荧光定量聚合酶链式反应(qRT-PCR)检测转染效果。
实验分为对照组、高糖组、高糖+Gly组、高糖+pcDNA3.1空载体组、高糖+pcDNA3.1-HMGB1组、高糖+Gly+pcDNA3.1空载体组、高糖+Gly+pcDNA3.1-HMGB1组。对照组、高糖组将未转染的H9c2细胞分别用低糖(5.5 mmol/L)DMEM培养基、高糖(35 mmol/L)DMEM培养基重悬[10];高糖+Gly组将未转染的H9c2细胞用含20 μmol/L Gly[11]的高糖DMEM培养基重悬;高糖+pcDNA3.1空载体组、高糖+pcDNA3.1-HMGB1组、高糖+Gly+pcDNA3.1空载体组、高糖+Gly+pcDNA3.1-HMGB1组将转染pcDNA3.1空载体、pcDNA3.1-HMGB1的H9c2细胞分别用高糖DMEM培养基、含20 μmol/L Gly的高糖DMEM培养基重悬。
1.3 CCK-8法检测各组H9c2细胞活力
将各组H9c2细胞以1×104个/孔接种至96孔板内,培养48 h后加入CCK-8试剂并继续培养2 h,借助Thermo MultiskanTMFC酶标仪测定450 nm波长处的吸光度(OD)。细胞活力与OD450值成正比。
1.4 Annexin V-FITC/PI法检测各组H9c2细胞凋亡率
将各组H9c2细胞以5×104个/孔接种至24孔板内,培养48 h后收集。将H9c2细胞转移至流式管内(100 μL中2×105个),加入5 μL Annexin V-FITC,4 ℃孵育15 min,而后加入5 μL的PI,4 ℃孵育5 min,借助BD FACSCantoⅡ流式细胞仪检测细胞凋亡率。
1.5 透射电镜观察各组H9c2细胞自噬
细胞培养处理同1.4,将H9c2细胞依次经戊二醛固定2 h、锇酸固定2 h,磷酸缓冲液洗后进行乙醇梯度脱水,而后进行包埋、超薄切片(60~80 nm),醋酸铀、柠檬酸铅各染色15 min,于低压小型透射电镜(LVEM)下观察并分析。
1.6 蛋白免疫印记法(Western Blot)检测凋亡和自噬相关蛋白表达情况
细胞培养处理同1.4,加入RIPA裂解液提取总蛋白,BCA法测定浓度后进行定量、变性,随后进行凝胶电泳分离蛋白、转膜、封闭,孵育兔源一抗anti-Bax、anti-LC3、anti-Beclin-1和anti-GAPDH(4 ℃过夜),次日孵育山羊抗兔二抗(室温2 h),最后滴加增强化学发光试剂(enhanced chemiluminescence,ECL),置于Tocan360凝胶成像分析系统中拍照,借助Image-Pro Plus 6.0软件进行灰度分析。
1.7 H9c2细胞IL-1β、TNF-α及ROS水平检测
细胞培养处理同1.4,收集培养上清,利用ELISA试剂盒测定上清中IL-1β、TNF-α水平;而后向各孔中加入无血清培养液稀释的DCFH-DA荧光探针(终浓度10 μmol/L),37 ℃孵育20 min后收集细胞,借助BD FACSCanto Ⅱ流式细胞仪检测平均荧光强度。ROS水平与平均荧光强度成正比。
1.8 qRT-PCR检测各组H9c2细胞HMGB1、RAGE mRNA表达水平
细胞培养处理同1.4,加入Trizol提取总RNA,经反转录合成cDNA,而后利用ABI7500 qRT-PCR仪扩增cDNA,以甘油醛-3-磷酸脱氢酶(GAPDH)为内参,采用2-△△CT法分析HMGB1、RAGE表达水平。引物序列:HMGB1正向引物为5′-CTAGCCCTGTCCTGGTGGTATT-3′,反向引物为5′-CCAATTTACAACCCCCAGACTGT-3′;RAGE正向引物为5′-AGGAAAGCCCTCCTGTCAACA-3′,反向引物为5′-CACAGAGCCTGCAGCTTGTC-3′;GAPDH正向引物为5′-GAAGGTCGGTGTGAACGGATTTG-3′,反向引物为5′-CATGTAGACCATGTAGTTGAGGTCA-3′。
1.9 统计学处理
采用SPSS 25.0软件进行统计学分析。符合正态分布的定量资料以均数±标准差(x±s)表示,多组间比较行One-way ANOVA分析,进一步两两比较行SNK-q检验。以P<0.05为差异有统计学意义。
2 结 果
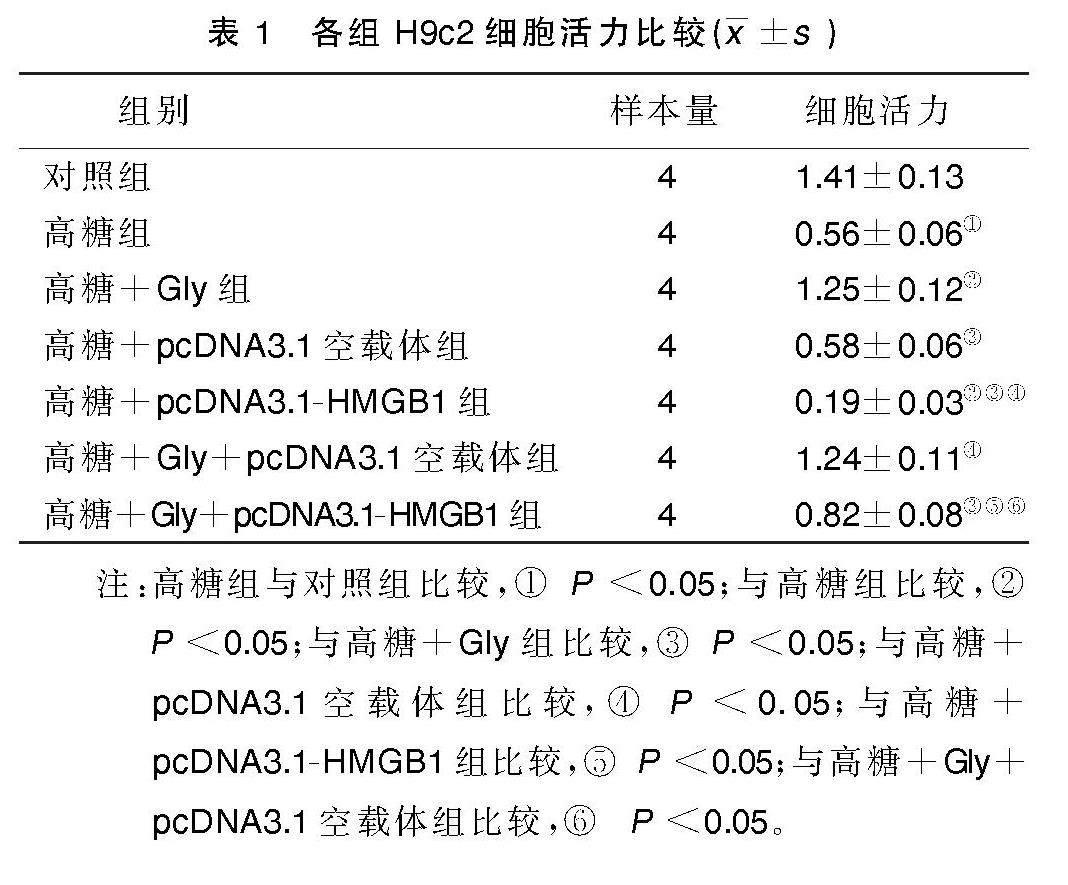
2.1 各组H9c2细胞活力比较
与对照组比较,高糖组H9c2细胞活力下降(P<0.05);与高糖组比较,高糖+Gly组H9c2细胞活力升高(P<0.05),高糖+pcDNA3.1-HMGB1组H9c2细胞活力下降(P<0.05);与高糖+Gly组比较,高糖+Gly+pcDNA3.1-HMGB1组H9c2细胞活力下降(P<0.05)。详见表1。
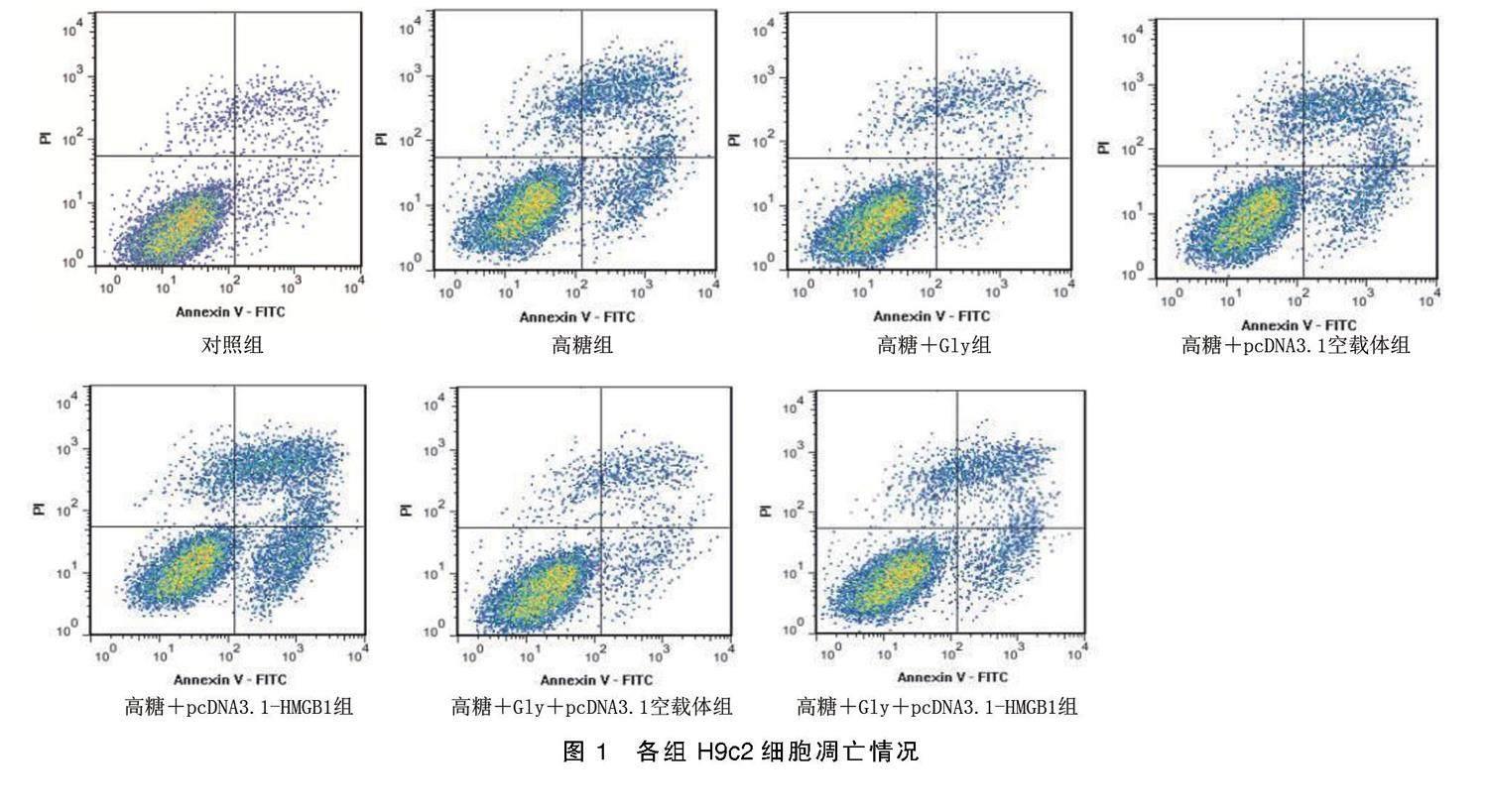
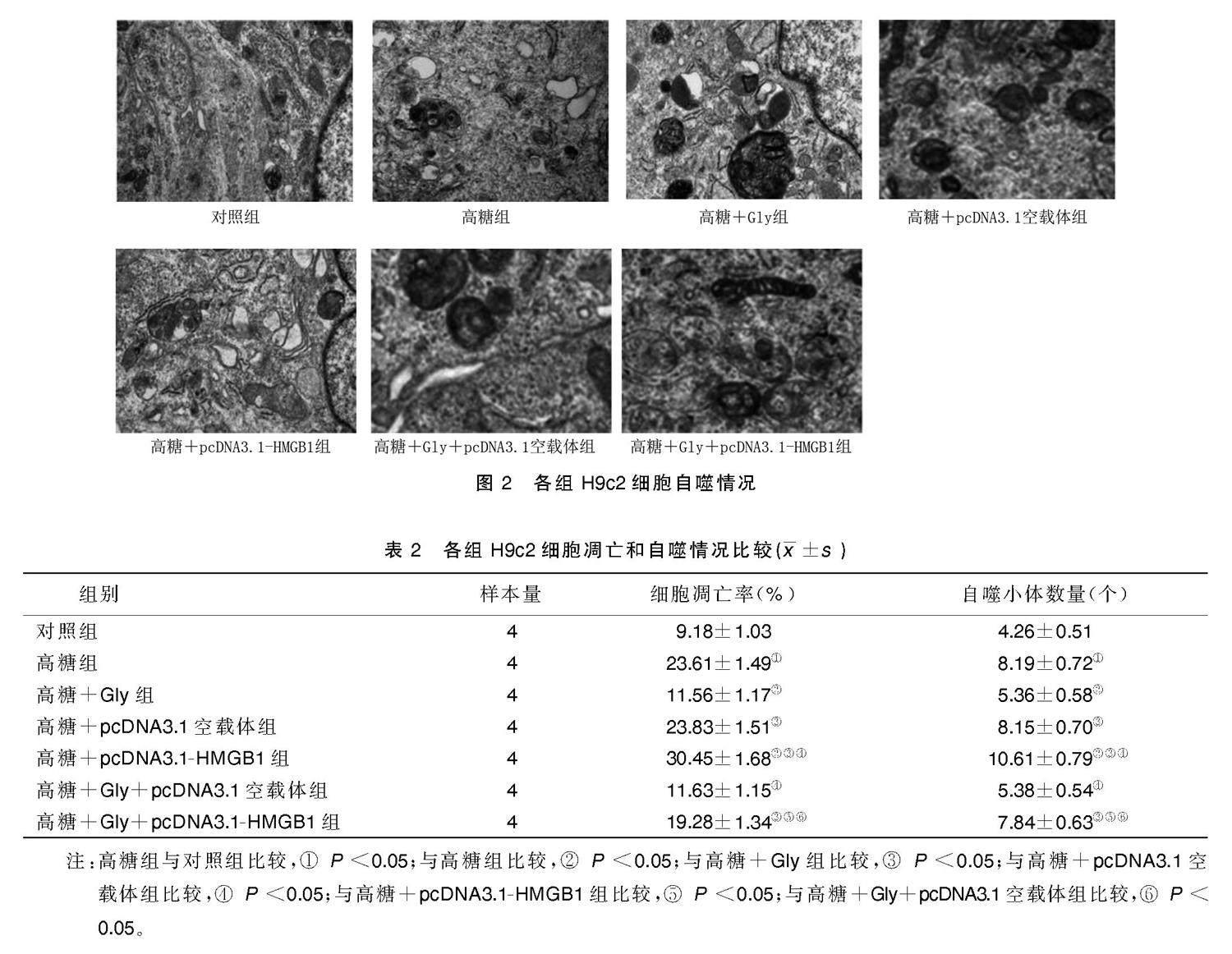
2.2 各组H9c2细胞凋亡和自噬情况比较
与对照组比较,高糖组H9c2细胞凋亡率、自噬小体数量增加(P<0.05);与高糖组比较,高糖+Gly组H9c2细胞凋亡率、自噬小体数量减少(P<0.05),高糖+pcDNA3.1-HMGB1组H9c2细胞凋亡率、自噬小体数量增加(P<0.05);与高糖+Gly组比较,高糖+Gly+pcDNA3.1-HMGB1组H9c2细胞凋亡率、自噬小体数量增加(P<0.05)。详见图1、图2、表2。
2.3 各组H9c2细胞凋亡和自噬相关蛋白表达情况
与对照组比较,高糖组H9c2细胞Bax、LC3、Beclin-1蛋白表达增加(P<0.05);与高糖组比较,高糖+Gly组H9c2细胞Bax、LC3、Beclin-1蛋白表达减少(P<0.05),高糖+pcDNA3.1-HMGB1组H9c2细胞Bax、LC3、Beclin-1蛋白表达增加(P<0.05);与高糖+Gly组比较,高糖+Gly+pcDNA3.1-HMGB1组H9c2细胞Bax、LC3、Beclin-1蛋白表达增加(P<0.05)。详见表3、图3。
2.4 各组H9c2细胞IL-1β、TNF-α及ROS水平比较
与对照组比较,高糖组H9c2细胞IL-1β、TNF-α及ROS水平增加(P<0.05);与高糖组比较,高糖+Gly组H9c2细胞IL-1β、TNF-α及ROS水平减少(P<0.05),高糖+pcDNA3.1-HMGB1组H9c2细胞IL-1β、TNF-α及ROS水平增加(P<0.05);与高糖+Gly组比较,高糖+Gly+pcDNA3.1-HMGB1组H9c2细胞IL-1β、TNF-α及ROS水平增加(P<0.05)。详见表4。
2.5 各组H9c2细胞HMGB1、RAGE mRNA表达水平比较
与对照组比较,高糖组H9c2细胞HMGB1、RAGE mRNA表达增加(P<0.05);与高糖组比较,高糖+Gly组H9c2细胞HMGB1、RAGE mRNA表达减少(P<0.05),高糖+pcDNA3.1-HMGB1组H9c2细胞HMGB1、RAGE mRNA表达增加(P<0.05);与高糖+Gly组比较,高糖+Gly+pcDNA3.1-HMGB1组H9c2细胞HMGB1、RAGE mRNA表达增加(P<0.05)。详见表5。
3 讨 论
DCM是一种独立于其他疾病的心脏损伤,由糖尿病本身引起,发病早期表现为心室舒张功能异常,后期表现为收缩功能障碍,若治疗不当,可发展为心力衰竭、心律失常、心源性休克甚至猝死[12]。据报道,DCM的发病机制涉及糖脂代谢紊乱、氧化应激、炎症反应、心肌钙调节机制受损等多种途径,其中炎症和氧化应激反应日益受到关注[13]。高血糖可促进ROS和炎性因子的过量产生,进而诱导心肌细胞凋亡和心功能损伤[14-15]。本研究中,H9c2细胞经高糖处理后,其IL-1β、TNF-α、ROS水平明显升高,同时细胞活力下降,细胞凋亡率、自噬小体数量及自噬、凋亡相关蛋白Bax、LC3、Beclin-1表达增加,表明高糖诱导H9c2细胞发生了炎症氧化应激反应,促进其凋亡和自噬。
Gly是甘草的主要活性成分之一,在各种疾病模型中,Gly已显示出抗炎和抗氧化应激的巨大潜力。Gly可通过抑制炎症反应减轻偶氮氧甲烷/葡聚糖硫酸钠诱导的结直肠癌小鼠的癌变[16]。Gly可通过抑制炎症和氧化应激反应改善Ⅱ型胶原诱导的关节炎症[17]。此外,Xu等[18]研究发现Gly可通过调节炎症和氧化状态改善心肌缺血损伤,其作用机制可能与激活核因子E2相关因子2/血红素加氧酶1、抑制核因子κB信号通路有关。本研结果显示,与高糖组比较,高糖+Gly组H9c2细胞IL-1β、TNF-α、ROS水平下降,同时细胞活力升高,凋亡和自噬现象好转,表明Gly能够减轻高糖诱导的H9c2细胞炎症和氧化应激反应,抑制其凋亡和自噬。
炎症和氧化应激反应过程涉及诸多信号通路的调节,其中HMGB1/RAGE发挥着关键性作用,HMGB1是一种因在凝胶电泳时迁移速度快而得名的高度保守的核蛋白,当受到外界刺激时HMGB1被释放到胞外,与膜受体RAGE相结合而促进多种炎性因子的表达,诱导或加重炎症和氧化应激反应[19]。研究显示,HMGB1/RAGE信号通路参与肺部疾病[20]、风湿性疾病[21]、脓毒症[22]、糖尿病及其相关并发症[6-7]等多种疾病的病理过程。本研究发现,H9c2细胞经高糖处理后HMGB1、RAGE表达增加,且过表达HMGB1后,H9c2细胞中IL-1β、TNF-α、ROS水平升高,细胞活力下降,凋亡和自噬现象加重,表明HMGB1/RAGE信号通路参与了高糖诱导的H9c2细胞损伤。为了探究抑制HMGB1/RAGE信号通路是否为Gly减轻高糖诱导的H9c2细胞损伤的潜在机制,首先检测了高糖致损伤的H9c2细胞在经Gly干预后其HMGB1、RAGE表达的差异,结果显示HMGB1、RAGE表达减少;然后在Gly干预的同时通过转染使HMGB1过表达,结果发现,Gly减轻高糖诱导的H9c2细胞炎症和氧化应激反应,抑制其凋亡和自噬的作用被削弱,上述结果综合表明抑制HMGB1/RAGE信号通路为Gly的潜在机制。
综上所述,Gly能够减轻高糖诱导的H9c2细胞炎症和氧化应激反应,抑制其凋亡和自噬,可能与抑制HMGB1/RAGE信号通路有关。
参考文献:
[1] MURTAZA G,VIRK H U H,KHALID M,et al.Diabetic cardiomyopathy--a comprehensive updated review[J].Progress in Cardiovascular Diseases,2019,62(4):315-326.
[2] ABUKHALIL M H,ALTHUNIBAT O Y,ALADAILEH S H,et al.Galangin attenuates diabetic cardiomyopathy through modulating oxidative stress,inflammation and apoptosis in rats[J].Biomedecine & Pharmacotherapie,2021,138:111410.
[3] ZHU Y B,QIAN X,LI J J,et al.Astragaloside-IV protects H9C2(2-1) cardiomyocytes from high glucose-induced injury via miR-34a-mediated autophagy pathway[J].Artificial Cells,Nanomedicine,and Biotechnology,2019,47(1):4172-4181.
[4] 李林霏,毛福英,李斯琦,等.甘草甜素的药理活性、作用机制及其应用进展[J].中华中医药学刊,2022,40(1):242-247.
[5] YUAN Y G,LI B,PENG W Z,et al.Protective effect of glycyrrhizin on coronary microembolization-induced myocardial dysfunction in rats[J].Pharmacology Research & Perspectives,2021,9(1):e00714.
[6] BEHL T,SHARMA E,SEHGAL A,et al.Expatiating the molecular approaches of HMGB1 in diabetes mellitus:highlighting signalling pathways via RAGE and TLRs[J].Molecular Biology Reports,2021,48(2):1869-1881.
[7] STEINLE J J.Role of HMGB1 signaling in the inflammatory process in diabetic retinopathy[J].Cellular Signalling,2020,73:109687.
[8] WANG Y,ZHANG Y,PENG G,et al.Glycyrrhizin ameliorates atopic dermatitis-like symptoms through inhibition of HMGB1[J].International Immunopharmacology,2018,60:9-17.
[9] MU S W,DANG Y,FAN Y C,et al.Effect of HMGB1 and RAGE on brain injury and the protective mechanism of glycyrrhizin in intracranial-sinus occlusion followed by mechanical thrombectomy recanalization[J].International Journal of Molecular Medicine,2019,44(3):813-822.
[10] 孔建强,邴淼,汪琼,等.右美托咪定调控miR-126对高糖诱导的心肌细胞氧化应激及凋亡的影响[J].西部医学,2021,33(3):336-341.
[11] LAI T F,SHEN Y,CHEN C C,et al.Glycyrrhizic acid ameliorates myocardial ischemia-reperfusion injury in rats through inhibiting endoplasmic reticulum stress[J].European Journal of Pharmacology,2021,908:174353.
[12] DILLMANN W H.Diabetic cardiomyopathy[J].Circulation Research,2019,124(8):1160-1162.
[13] RITCHIE R H,ABEL E D.Basic mechanisms of diabetic heart disease[J].Circulation Research,2020,126(11):1501-1525.
[14] YIN Q,LI Z D,LU S Y.Knockdown of ILK alleviates high glucose-induced damage of H9c2 cells through TLR4/MyD88/NF-κB pathway[J].Disease Markers,2022,2022:6205190.
[15] YANG Z,WU Y,WANG L G,et al.Prokineticin 2 (PK2) rescues cardiomyocytes from high glucose/high palmitic acid-induced damage by regulating the AKT/GSK3β pathway in vitro[J].Oxidative Medicine and Cellular Longevity,2020,2020:3163629.
[16] WANG G F,HIRAMOTO K,MA N,et al.Glycyrrhizin attenuates carcinogenesis by inhibiting the inflammatory response in a murine model of colorectal cancer[J].International Journal of Molecular Sciences,2021,22(5):2609.
[17] SHAFIK N M,EL-ESAWY R O,MOHAMED D A,et al.Regenerative effects of glycyrrhizin and/or platelet rich plasma on type-Ⅱ collagen induced arthritis:targeting autophay machinery markers,inflammation and oxidative stress[J].Archives of Biochemistry and Biophysics,2019,675:108095.
[18] XU C L,LIANG C H,SUN W X,et al.Glycyrrhizic acid ameliorates myocardial ischemic injury by the regulation of inflammation and oxidative state[J].Drug Design,Development and Therapy,2018,12:1311-1319.
[19] ZHANG C,DONG H,CHEN F,et al.The HMGB1-RAGE/TLR-TNF-α signaling pathway may contribute to kidney injury induced by hypoxia[J].Exp Ther Med,2019,17(1):17-26.
[20] ZHANG Y,ZHANG M,WANG C Y,et al.Ketamine alleviates LPS induced lung injury by inhibiting HMGB1-RAGE level[J].European Review for Medical and Pharmacological Sciences,2018,22(6):1830-1836.
[21] SHI J,XU H,CAVAGNARO M J,et al.Blocking HMGB1/RAGE signaling by berberine alleviates A1 astrocyte and attenuates sepsis-associated encephalopathy[J].Frontiers in Pharmacology,2021,12:760186.
[22] LIN S S,YUAN L J,NIU C C,et al.Hyperbaric oxygen inhibits the HMGB1/RAGE signaling pathway by upregulating miR-107 expression in human osteoarthritic chondrocytes[J].Osteoarthritis and Cartilage,2019,27(9):1372-1381.
(收稿日期:2022-12-05)
(本文编辑郭怀印)



