Deokho Lee,Yohei Tomita,Kazuno Negishi,Toshihide Kurihara
Ischemic retinopathy is a retinal disease caused by ischemic attacks.Ischemia is a common pathologic mechanism in various retinal disorders and diseases such as age-related macular degeneration,diabetic retinopathy,glaucoma,or vascular occlusion (Osborne et al.,2004).Although various murine models have been developed to understand a series of metabolic mechanisms induced by retinal ischemia and further used to test promising therapeutics,effective treatment in ischemic retinopathy has not been clearly suggested.This is associated with the notion that the contributing pathologic metabolic pathways might be enormously complex.
Over the past few years,our group has been targeting peroxisome proliferator-activated receptor α (PPARα) as a promising therapeutic receptor in eye diseases.PPARα is one of the ligand-activated transcription factors belonging,together with the other PPARγ and PPARβ/δ.PPARα is expressed in metabolically active tissues such as the liver,heart,skeletal muscle,intestinal mucosa,brown adipose,brain,and eye (Bougarne et al.,2018).As its activation is involved in a variety of metabolic functions,PPARα modulation has emerged as a novel therapeutic concept for treating central nervous system diseases (Lee et al.,2021a).Furthermore,PPARα activation by fenofibrate treatment has shown beneficial effects on diabetic retinopathy in two large clinical trials;the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye studies.In this regard,we speculated that PPARα activation might exert therapeutic effects on ischemic retinopathy.
By the time we planned the experimental scheme for therapeutic roles of PPARα activation in ischemic retinopathy,we had chance to use pemafibrate,a potent selective PPARα modulator(SPPARMα).As concerns on the use of fenofibrate has been gradually reported in clinical cases of adverse events associated with renal dysfunction(Kostapanos et al.,2013),pemafibrate has been designed to selectively and potently activate PPARα,which could be safer than other PPARα agonists.In our first experimental study,we used both drugs (pemafibrate and fenofibrate)to examine therapeutic roles on pathological neovascularization in a mouse model of oxygeninduced retinopathy (Tomita et al.,2019).We found that pemafibrate treatment significantly suppressed pathological neovascularization while fenofibrate treatment only showed a suppressing tendency without stati stical significance.Although we only selected one concentration (pemafibrate:0.3 mg/kg per day;fenofibrate: 10 mg/kg per day) for each group,pemafibrate showed a positive outcome even at a low concentration.Pemafibrate treatment increased serum fibroblast growth factor 21 (FGF21) levels and decreased retinal hypoxia-inducible factor-1α (HIF-1α)immunoreactivity (a master regulator to control vascular endothelial growth factor,VEGF) and retinalVegfamRNA expression (one of the major pathological molecules for developing neovascularization).Furthermore,the suppressive effect on HIF activity was explained with longacting FGF21 molecule (PF-05231023) treatment to a 661W retinal cell line under a HIF luciferase assay condition,rather than pemafibrate treatment.
Next,we aimed to apply the pemafibrate therapy to experimental diabetic retinopathy (Tomita et al.,2020).Pemafibrate administration mildly reduced blood glucose levels and protected against retinal dysfunction (reductions in the amplitudes of oscillatory potenti als) with preservation of retinal synaptophysin expression (one of the presynaptic vesicle proteins) in streptozotocin (STZ)-induced diabetic mice.Similar to the oxygen-induced retinopathy model,the STZ model also showed increased serum FGF21 levels by pemafibrate administration.This outcome seemed highly consistent from young mice to adult mice.As STZ-induced diabetic mice had limitations in inducing ischemic retinopathy (which is different from the human diabetic condition),we induced an ischemic attack in adult mice using surgical unilateral common carotid artery occlusion(UCCAO) (Lee et al.,2021b).Similar to STZ-induced diabetic mice,pemafibrate administration showed a preservation of retinal function in UCCAOinduced ocular ischemic mice.Furthermore,we found that UCCAO-induced pathological retinal gliosis was reduced by pemafibrate administration.As FGF21 has been recently suggested to modulate gliosis in Müller cells (Fu et al.,2021),we speculated that increases in serum FGF21 levels by pemafibrate treatment might have protective roles in the ischemic retina by modulating retinal gliosis.Although STZ/UCCAOinduced diabetic ischemic mice have not yet been developed,therapeutic roles of pemafibrate on diabetic ischemic retinopathy are desired to be tested for the further study,which can be more relevant to human diabetic conditions.
Recently,we attempted to examine the therapeutic roles of pemafibrate in a mouse model of retinal ischemia/reperfusion injury through transient elevation of intraocular pressure(Lee et al.,2022).As retinal ischemia/reperfusion injury induces retinal ganglion cell (RGC) loss,its model could mimic one of the conditions for acute glaucoma.Retinal ischemia/reperfusion injury-induced RGC death and retinal dysfunction were prevented by pemafibrate administration.Pathologic inflammation (especially,retinal microglial activation and reactive gliosis) was also reduced.At the molecular level,retinal HIF-1α and its downstreamVegfamRNA expressions were reduced by pemafibrate administration.Taken together,we found that the pemafibrate therapy could work on various types of ischemic retinopathy.
Promising pemafibrate therapy for ocular diseases has been conducted not only by our group but also other groups.Instead of using mice in our studies,rats were used in their studies.Increases in retinal vascular leakage and leukostasis were reduced by pemafibrate administration in STZ-induced diabetic rats (Shiono et al.,2020).Furthermore,its effect was explained by upregulation of thrombomodulin expression (an important factor for maintaining vascular homeostasis) by pemafibrate treatment using the small interfering RNA knockdown system.A rat model of N-methyl-D-aspartateinduced RGC death was also used to examine the therapeutic effects of pemafibrate (Fujita et al.,2021).RGC loss was prevented by pemafibrate administration,and its effect was explained by a reduction in phosphorylated c-Jun expression(associated with the expression of apoptosisrelated genes) in the retina.Furthermore,they found that phosphorylated c-Jun was labeled with dying RGCs in their experimental conditions.Taken together,the therapeutic effects of pemafibrate on retinal diseases have been well-stacked in preclinical studies (Figure 1).
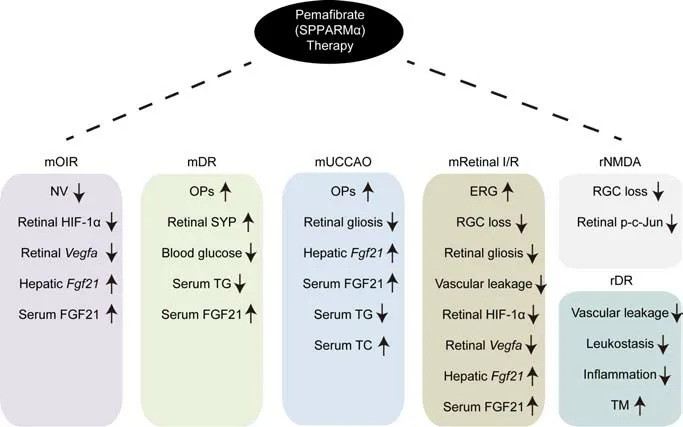
Figure 1|Summary of therapeutic roles of pemafibrate on retinopathy in preclinical studies.
Limitations and future directions:To date,the therapeutic strategy was mostly conducted with oral administration of pemafibrate.Even though applying the oral pemafibrate therapy is reasonable for preclinical studies (as pemafibrate has been orally treated in clinics),understanding the direct mode of action of pemafibrate to the eye needs more evidence;does pemafibrate affect the retina,retinal pigment epithelium,choroid,or the other parts of the eye? In fact,from a different point of view,systemic metabolic boosting by oral administration of pemafibrate might also contribute to ocular protection in that metabolic dysregulation is highly connected with ocular diseases (especially,diabetic retinopathy).This should be further studied to clarify the role of pemafibrate in the body.Recently,topical application of PPARα activator,such as eye drops,has been attempted on retinal diseases (Hanaguri et al.,2022).The therapeutic effects could be locally applied to the damaging sites rather than the oral administration.In this regard,topical administration of pemafibrate is also desirable to be developed for a new type of vision therapy.Even though more preclinical studies are needed,the emerging evidence suggests that pemafibrate,SPPARMα,is a promising novel treatment for various types of ischemic retinopathy.
This work was supported by the KAKENHI,Nos.15K10881 and 18K09424 (to TK) and JST SPRING,No.JPMJSP2123 (to DL).
Deokho Lee,Yohei Tomita,
Kazuno Negishi,Toshihide Kurihara*
Department of Ophthalmology,Keio University School of Medicine,Shinanomachi,Shinjuku-ku,Japan
*Correspondence to:Toshihide Kurihara,MD,PhD,kurihara@28keio.jp.
https://orcid.org/0000-0002-5457-2720(Toshihide Kurihara)
Date of submission:August 28,2022
Date of decision:October 10,2022
Date of acceptance:October 15,2022
Date of web publication:November 9,2022
https://doi.org/10.4103/1673-5374.360319
How to cite this article:Lee D,Tomita Y,Negishi K,Kurihara T (2023) Pemafibrate,a potent selective peroxisome proliferator-activated receptor α modulator,a promising novel treatment for ischemic retinopathy? Neural Regen Res 18(7):1495-1496.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.



