周朝霞,张骥,赵媛,王肖潇,吕欢欢
摘要目的:探讨芍药苷对盐敏感性高血压(SSH)大鼠血压和血管内皮功能的影响及其相关作用机制。方法:将50只Dahl盐敏感大鼠随机分为正常对照组(Control组)、高盐组(SSH组)、芍药苷组(PF组)、磷脂酰肌醇-3-激酶(PI3K)/蛋白激酶B(AKT)/哺乳动物雷帕霉素靶蛋白(mTOR)信号通路激活剂组(740Y-P组)、芍药苷+740Y-P组(PF+740Y-P组),每组10只。各组大鼠进行4周给药干预。采用动物无创血压仪测量大鼠尾动脉收缩压、舒张压;酶联免疫吸附法(ELISA)测定大鼠血清内皮素-1(ET-1)、一氧化氮(NO)、血栓素B2(TXB2)水平;苏木精-伊红(HE)染色观察大鼠主动脉病理变化;免疫组织化学染色检测大鼠主动脉组织中内皮型一氧化氮合酶(eNOS)表达;蛋白质免疫印迹法(Western Blot)检测大鼠主动脉组织中PI3K/AKT/mTOR信号通路蛋白表达。结果:与Control组比较,SSH组和740Y-P组大鼠主动脉血管内皮不完整,部分血管内皮脱落,且内膜明显增厚、外膜有大量沉积物;PF组大鼠主动脉血管病理损伤较SSH组明显减轻;PF+740Y-P组大鼠主动脉血管病理损伤较740Y-P组明显减轻,但较PF组明显加重。与Control组比较,SSH组大鼠收缩压、舒张压、血清ET-1、TXB2水平均升高,血清NO水平降低(P<0.05);主动脉组织中eNOS表达水平降低,磷酸化(p)-PI3K/PI3K、p-AKT/AKT、p-mTOR/mTOR比值均升高(P<0.05)。与SSH组比较,PF组大鼠收缩压、舒张压、血清ET-1、TXB2水平均降低,血清NO水平升高(P<0.05);主动脉组织中eNOS表达水平升高,p-PI3K/PI3K、p-AKT/AKT、p-mTOR/mTOR比值均降低(P<0.05)。与PF组比较,PF+740Y-P组大鼠收缩压、舒张压、血清ET-1、TXB2水平均升高,血清NO水平降低(P<0.05);主动脉组织中eNOS表达水平降低,p-PI3K/PI3K、p-AKT/AKT、p-mTOR/mTOR比值均升高(P<0.05)。与740Y-P组比较,PF+740Y-P组大鼠收缩压、舒张压、血清ET-1、TXB2水平均降低,血清NO水平升高(P<0.05);主动脉组织中eNOS表达水平升高,p-PI3K/PI3K、p-AKT/AKT、p-mTOR/mTOR比值均降低(P<0.05)。结论:芍药苷可以有效降低SSH大鼠血压,并改善大鼠血管内皮功能,其作用机制可能与抑制PI3K/AKT/mTOR信号通路激活有关。
关键词盐敏感性高血压;芍药苷;血压;血管内皮功能;磷脂酰肌醇-3-激酶/蛋白激酶B/哺乳动物雷帕霉素靶蛋白信号通路;实验研究
doi:10.12102/j.issn.1672-1349.2024.08.009
Effects of Paeoniflora on Blood Pressure and Vascular Endothelial Function in Salt-sensitive Hypertensive Rats by Regulating PI3K/AKT/mTOR Signaling Pathway
ZHOU Zhaoxia, ZHANG Ji, ZHAO Yuan, WANG Xiaoxiao, LYU Huanhuan
Shaanxi Provincial People′s Hospital, Xi′an 710068, Shaanxi, China, E-mail: zhouzhaoxia2001@163.com
AbstractObjective:To observe the effect of Paeoniflorin on blood pressure and vascular endothelial function in salt-sensitive hypertensive(SSH) rats and its mechanism.Methods:Fifty Dahl salt-sensitive rats were randomly divided into normal control group,high salt group(SSH group),Paeoniformin group(PF group),Phosphatidylinositol-3-kinase(PI3K)/protein kinase B(AKT)/mammalian target of rapamycin(mTOR) signaling pathway activator group 740Y-P,paeoniformin +740Y -P group(PF+740Y-P group),with 10 rats in each group.The rats in each group were treated with drug intervention for 4 weeks.Rat tail artery systolic and diastolic blood pressure were measured by animal by animal non-invasive sphygmomanometer.The serum levels of endothelin-1(ET-1),nitric oxide(NO) and thromboxane B2(TXB2) were detected by enzyme-linked immunosorbent assay(ELISA).Hematoxylin-eosin(HE) staining was used to observe the pathological changes of aorta in rats.The protein expression of endothelial nitric oxide synthetase(eNOS) in rat aorta was detected by immunohistochemical staining.The expression of PI3K/AKT/mTOR signaling pathway in rat aorta was detected by Western Blot.Results:Compared with the Control group,the aortic endothelium in the SSH group and the 740Y-P group was incomplete,part of the vascular endothelium was detached,the intima was obviously proliferated,and there were large amounts of deposits in the outer membrane.The pathological damage of aorta vessels in the PF group was significantly less than that in SSH group.The pathological damage of aorta in the PF+740Y-P group significantly decreased than that in the 740Y-P group,but significantly aggravated than that in the PF group.Compared with the Control group,systolic blood pressure,diastolic blood pressure,serum ET-1 and TXB2 levels increased in SSH group,while serum NO level decreased(P<0.05).The expression level of eNOS in aortic tissues decreased,while the phosphorylation(p) -PI3K/PI3K,p-AKT/AKT and p-mTOR/mTOR increased(P<0.05).Compared with the PF group,systolic blood pressure,diastolic blood pressure,serum ET-1 and TXB2 levels in the PF+740Y-P group increased,while the serum NO level decreased(P<0.05).The expression level of eNOS in aortic tissues decreased,while the p-PI3K/PI3K,P-AKT/AKT and p-mTOR/mTOR increased(P<0.05).Compared with the 740Y-P group,systolic blood pressure,diastolic blood pressure,serum ET-1 and TXB2 levels in the PF+740Y-P group decreased,while the serum NO level increased(P<0.05).The expression level of eNOS in aortic tissues increased,and the p-PI3K/PI3K,p-AKT/AKT and p-mTOR/mTOR decreased(P<0.05).Conclusion:Paeoniflora could effectively reduce blood pressure and improve vascular endothelial function in SSH rats,and its mechanism might be related to inhibiting the activation of PI3K/AKT/mTOR signaling pathway.
Keywordssalt-sensitive hypertension; Paeoniflorin; blood pressure; vascular endothelial function; phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway; experimental study
盐敏感性高血压(salt-sensitive hypertension,SSH)是一种重要的、特殊类型的原发性高血压。现在生活习惯中高盐饮食导致血压升高,已成为心血管疾病及慢性肾病发生和发展过程中一个重要的独立危险因素[1]。尽管临床上控制高盐摄入量以及药物治疗降低血压的策略取得了一定的进展,但当前的治疗策略却不能彻底预防SSH进展引发的心血管、肾脏等损伤[2]。因此,探索开发防治SSH的新的有效治疗药物具有重要临床意义。内皮细胞可分泌血管扩张和收缩物质,在控制血管张力和血液流动方面起着重要作用,内皮功能障碍的特征是内皮细胞的作用向促血栓形成、促炎症和促收缩特性转变。在包括SSH在内的各种心血管疾病中都观察到血管内皮功能障碍的发生,靶向调节血管内皮功能可能为高血压及其相关心血管疾病提供新的治疗策略[3-4]。芍药苷(Paeoniflorin)是一种源自乳花芍药的天然产物,具有抗炎、抗肿瘤和抗凋亡等多种药理活性。研究证实,芍药苷在治疗心血管疾病方面具有显着疗效[5]。在急性心肌梗死大鼠模型中,芍药苷治疗减小了梗死面积并降低了肌酸激酶、乳酸脱氢酶等心肌损伤标志物水平[6]。在自发性高血压的压力超负荷模型以及脂多糖诱导的心功能不全动物模型中,芍药苷通过降低炎性因子水平减少心脏炎症并改善心脏功能[7-8]。然而,芍药苷对SSH的体内作用及其潜在机制在很大程度上仍然未知。因此,本研究观察芍药苷对高盐饮食诱导的SSH大鼠模型血压和血管内皮功能的影响。
1材料与方法
1.1材料
无特定病原体(SPF)级6周龄健康雄性Dahl盐敏感大鼠,体质量160~180 g,购自西安交通大学实验动物中心,动物合格证号:SCXK(陕)2020-001。大鼠在室温(23±2)℃、相对湿度(55±5)%、12 h光暗交替的SPF环境中饲养1周后开展实验。芍药苷、磷脂酰肌醇-3-激酶(PI3K)/蛋白激酶B(AKT)/哺乳动物雷帕霉素靶蛋白(mTOR)信号通路激活剂740Y-P均购自美国MCE公司;内皮素-1(endothelin-1,ET-1)、一氧化氮(nitric oxide,NO)、血栓素B2(thromboxane B2,TXB2)酶联免疫吸附法(ELISA)检测试剂盒购自南京建成生物工程研究所;苏木精-伊红(HE)染色试剂盒、免疫组织化学染色试剂盒购自上海捷瑞生物工程有限公司;内皮型一氧化氮合成酶(endothelial nitric oxide synthase,eNOS)、PI3K、磷酸化PI3K(phosphorylated PI3K,p-PI3K)、AKT、磷酸化AKT(phosphorylated AKT,p-AKT)、mTOR、磷酸化mTOR(phosphorylated mTOR,p-mTOR)、β-actin抗体和辣根过氧化物酶标记的山羊抗兔二抗购自英国Abcam公司。
1.2方法
1.2.1实验分组及模型建立
将大鼠随机分为正常对照组(Control组)、高盐组(SSH组)、芍药苷组(PF组)、PI3K/AKT/mTOR信号通路激活剂组(740Y-P组)、芍药苷+740Y-P组(PF+740Y-P组),每组10只。测量并记录大鼠基础血压。Control组大鼠给予6周含0.3%氯化钠(NaCl)的低盐饲料喂养。其余各组参照文献[9]方法进行造模:大鼠给予含8%NaCl的高盐饲料喂养,每周测1次血压,6周后,测量发现大鼠收缩压较基础血压升高大于10%且≥140 mmHg,即判定为造模成功。
1.2.2给药干预
造模成功后第2 天,根据参考文献[10]方法和预实验结果确定给药剂量进行给药干预。PF+740Y-P组大鼠以80 mg/kg芍药苷溶液每天灌胃1次,同时以0.02 mg/kg的740Y-P溶液每天腹腔注射1次;PF组大鼠以80 mg/kg的芍药苷溶液每天灌胃1次,同时以0.02 mg/kg剂量的生理盐水每天腹腔注射干预1次;740Y-P组大鼠以80 mg/kg剂量的生理盐水每天灌胃1次,同时以0.02 mg/kg的740Y-P溶液每天腹腔注射1次;Control组和SSH组大鼠以等量生理盐水每天灌胃及腹腔注射1次。连续干预4周。
1.2.3大鼠血压测量
采用BP-2000R2动物无创血压仪测量大鼠尾动脉收缩压、舒张压。每只大鼠于清醒安静状态下每周同一时间重复测量5次,取平均值作为该只大鼠本周血压。记录大鼠给药前、给药4周后的血压。
1.2.4血管内皮功能活性物质检测
末次给药结束后,大鼠禁食不禁水24 h,腹主动脉采血,分离血清,ELISA法测定血清ET-1、NO、TXB2水平。
1.2.5HE染色
取血后处死大鼠,分离胸主动脉血管,一部分保存于液氮中,一部分用4%多聚甲醛固定,制成4 μm厚的石蜡切片,进行HE染色观察主动脉病理变化。
1.2.6免疫组织化学染色
将大鼠主动脉石蜡切片置于60 ℃恒温箱中烘烤2 h,脱蜡、水化,3%过氧化氢溶液避光封闭10 min,磷酸缓冲盐溶液(PBS)洗涤,用10 μmol/L柠檬酸钠溶液进行修复抗原,PBS洗涤,滴加山羊血清封闭30 min,滴加eNOS抗体(1∶500)于4 ℃孵育过夜,PBS洗涤,滴加二抗37 ℃孵育2 h,PBS洗涤,用二氨基联苯胺(DAB)溶液显色,苏木精复染,脱水、透明、封片后,在光学显微镜下观察,阳性细胞呈棕色或棕褐色,采用Image Pro 5.0软件分析阳性区域光密度值(OD值)。
1.2.7蛋白质免疫印迹法(Western Blot)实验
用RIPA裂解缓冲液提取大鼠主动脉内皮组织中总蛋白,蛋白质含量用二喹啉甲酸法(BCA)试剂盒测定。将等量的蛋白质样品进行十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)分离,并转移至聚偏二氟乙烯(PVDF)膜上。用5%脱脂牛奶阻断膜2 h,洗膜,将膜在4 ℃下置于PI3K(1∶500)、p-PI3K(1∶500)、AKT(1∶500)、p-AKT(1∶500)、mTOR(1∶800)、p-mTOR(1∶500)、β-actin(1∶1 000)一抗中孵育过夜,洗膜,将膜与二抗(1∶2 000)室温孵育2 h,洗膜,在膜上滴加增强化学发光试剂,在凝胶成像系统中显影。以β-actin为内参,用Image J软件进行蛋白条带灰度值分析。
1.3统计学处理
采用SPSS 25.0软件进行统计学分析,符合正态分布的定量资料以均数±标准差(x±s)表示,多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验,以P<0.05为差异有统计学意义。
2结果
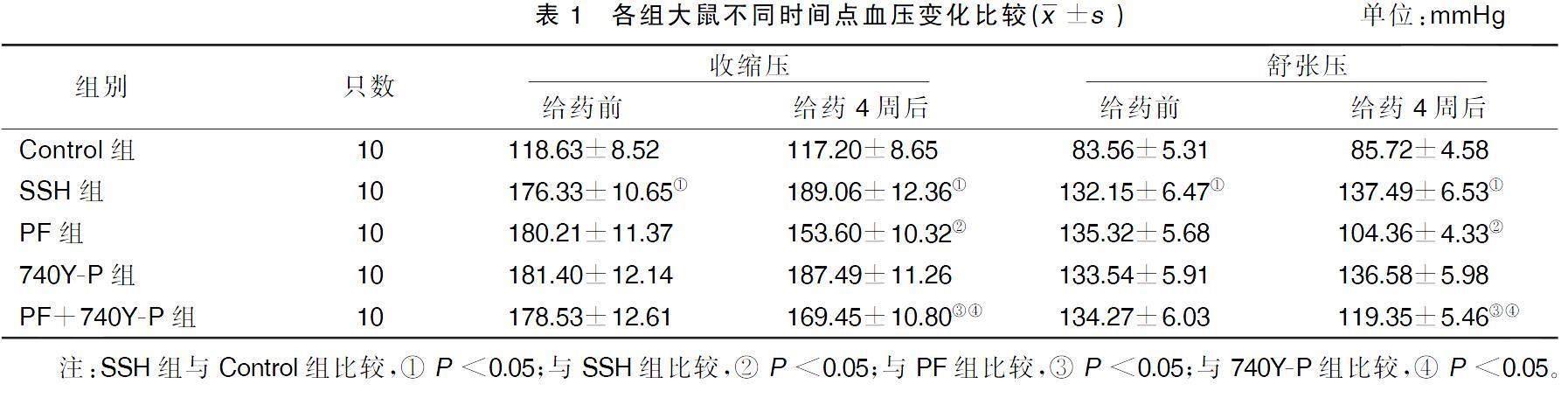
2.1各组大鼠不同时间点血压变化比较
给药前,与Control组比较,SSH组大鼠收缩压和舒张压均显着升高(P<0.05);与SSH组比较,PF组、740Y-P组和PF+740Y-P组大鼠收缩压和舒张压比较差异均无统计学意义(P>0.05)。给药4周后,与Control组比较,SSH组大鼠收缩压和舒张压均显着升高(P<0.05);与SSH组比较,PF组大鼠收缩压和舒张压均显着降低(P<0.05),740Y-P组大鼠收缩压和舒张压变化差异均无统计学意义(P>0.05);与PF组比较,PF+740Y-P组大鼠收缩压和舒张压均显着升高(P<0.05);与740Y-P组比较,PF+740Y-P组大鼠收缩压和舒张压均显着降低(P<0.05)。详见表1。
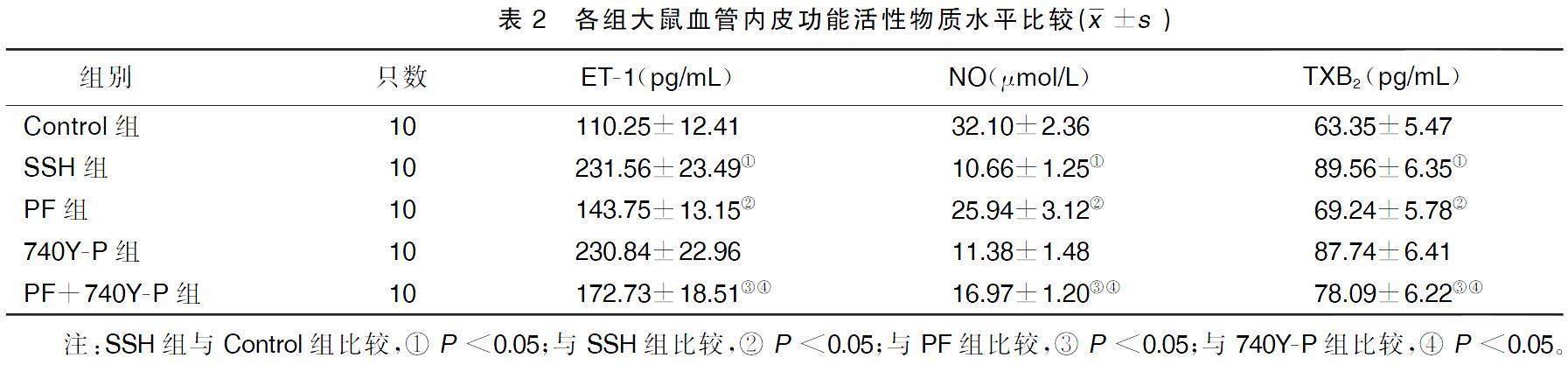
2.2各组大鼠血管内皮功能活性物质水平比较
与Control组比较,SSH组大鼠血清ET-1、TXB2水平均显着升高,NO水平显着降低(P<0.05);与SSH组比较,PF组大鼠血清ET-1、TXB2水平均显着降低,NO水平显着升高(P<0.05),740Y-P组大鼠血清ET-1、TXB2、NO水平变化差异均无统计学意义(P>0.05);与PF组比较,PF+740Y-P组大鼠血清ET-1、TXB2水平均显着升高,NO水平显着降低(P<0.05);与740Y-P组比较,PF+740Y-P组大鼠血清ET-1、TXB2水平均显着降低,NO水平显着升高(P<0.05)。详见表2。
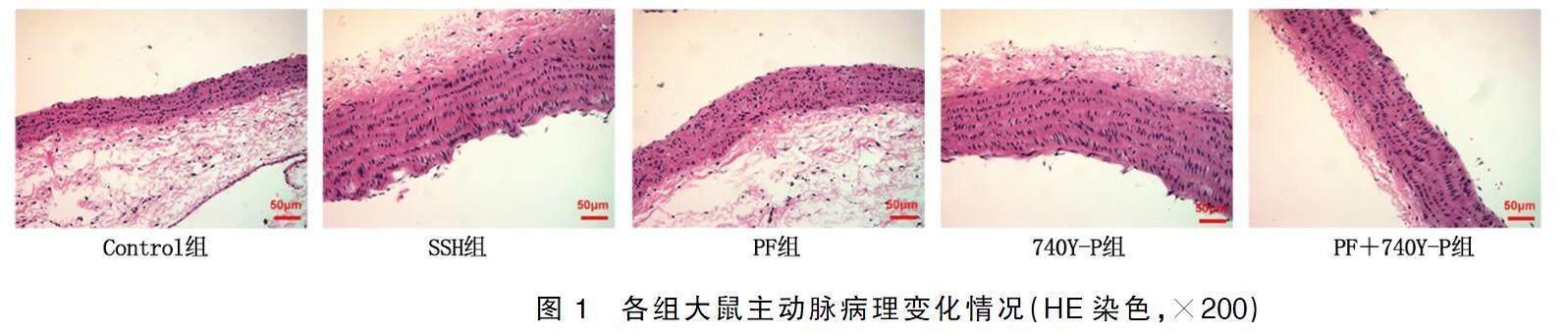
2.3各组大鼠主动脉病理变化情况
Control组大鼠主动脉血管内皮完整,表面光滑,呈梭形的内皮细胞单层紧密排列,结构正常;SSH组和740Y-P组大鼠主动脉血管内皮不完整,部分血管内皮脱落,细胞缺失处内膜不光滑,且内膜明显增厚,外膜有大量沉积物;PF组大鼠主动脉血管内皮脱落、内膜增厚及外膜沉积情况较SSH组均明显减轻;PF+740Y-P组大鼠主动脉血管病理损伤较740Y-P组明显减轻,但较PF组明显加重。详见图1。
2.4各组大鼠主动脉组织中eNOS表达比较
与Control组比较,SSH组大鼠主动脉组织中eNOS表达水平显着降低(P<0.05);与SSH组比较,PF组大鼠主动脉组织中eNOS表达水平显着升高(P<0.05),740Y-P组大鼠主动脉组织中eNOS表达水平变化差异无统计学意义(P>0.05);与PF组比较,PF+740Y-P组大鼠主动脉组织中eNOS表达水平显着降低(P<0.05);与740Y-P组比较,PF+740Y-P组大鼠主动脉组织中eNOS表达水平显着升高(P<0.05)。详见图2、图3。
2.5各组大鼠主动脉组织中PI3K/AKT/mTOR信号通路相关蛋白表达比较
与Control组比较,SSH组大鼠主动脉组织中p-PI3K/PI3K、p-AKT/AKT、p-mTOR/mTOR比值均显着升高(P<0.05);与SSH组比较,PF组大鼠主动脉组织中p-PI3K/PI3K、p-AKT/AKT、p-mTOR/mTOR比值均显着降低(P<0.05),740Y-P组大鼠主动脉组织中p-PI3K/PI3K、p-AKT/AKT、p-mTOR/mTOR比值均显着升高(P<0.05);与PF组比较,PF+740Y-P组大鼠主动脉组织中p-PI3K/PI3K、p-AKT/AKT、p-mTOR/mTOR比值均显着升高(P<0.05);与740Y-P组比较,PF+740Y-P组大鼠主动脉组织中p-PI3K/PI3K、p-AKT/AKT、p-mTOR/mTOR比值均显着降低(P<0.05)。详见图4、图5。
3讨论
芍药苷是一种单萜苷类化合物,具有重要的药用价值。研究证明,芍药苷可通过缓解高血压减轻靶器官的损伤[11]。Li等[12]研究报道,芍药苷和美托洛尔联合作用可改善自发性高血压大鼠的微循环,减轻内皮功能障碍,具有协同抗高血压的作用。Wu等[13]报道,芍药苷可以促进妊娠高血压大鼠沉默信息调节因子2相关酶1(SIRT1)和NO/eNOS的表达,抑制诱导型一氧化氮合成酶(iNOS)的产生,改善血管内皮细胞损伤,从而缓解妊娠高血压的发展。此外,芍药苷还可通过抑制内皮-间充质转化、抑制肺动脉周围炎性细胞浸润减轻肺动脉高压[14-15]。本研究探究芍药苷对SSH大鼠血压的影响,结果显示,芍药苷干预能够有效降低SSH大鼠收缩压和舒张压,表明芍药苷在SSH中具有显着的抗血压活性。
SSH血压升高与血管内皮功能受损和血管收缩反应异常增强有关[16]。内皮细胞通过合成和释放一系列内皮衍生的舒张因子,包括前列腺素、NO、内皮依赖性超极化因子以及血管紧张素Ⅱ、ET-1和血栓素等血管活性肽物质,在调节血管张力方面发挥着重要作用,内皮功能障碍主要是由内皮衍生的放松因子的产生或作用减少引起的[4]。研究报道,铁皮石斛通过降低血液中ET-1和TXB2含量,增加前列环素和NO含量以及胸主动脉eNOS表达,保护血管内皮功能,降低高糖高脂及复方酒精诱导高血压大鼠的收缩压和平均动脉压,最终抵抗高血压[17]。Xie等[18]研究发现,芍药苷通过调节TXB2、eNOS和ET-1等血管内皮活性物质水平发挥抗血栓作用。并且一些研究已经证明了芍药苷对各种实验疾病模型中血管内皮功能的调节作用。芍药苷通过促进血管内皮生长因子(vascular endothelial growth factor,VEGF)A上调缓解先兆子痫中可溶性FMS样酪氨酸激酶1和可溶性分泌素引起的内皮功能障碍[19]。芍药苷通过抗氧化和抗炎作用在波动性高血糖诱导的血管内皮损伤中发挥保护作用[20]。此外,芍药苷通过抑制缺氧诱导因子-1α(hypoxia-inducible factor-1α,HIF-1α)/VEGF/信号转导和转录激活因子3(STAT3)途径,阻止了缺氧诱导的人视网膜毛细血管内皮细胞血管生成,最终减轻了视网膜静脉阻塞小鼠的视网膜病变[21]。本研究结果显示,芍药苷可显着降低SSH大鼠血清ET-1、TXB2水平,升高血清NO水平,同时升高大鼠主动脉组织中eNOS表达水平。此外,主动脉病理形态学观察显示,芍药苷可减轻SSH大鼠主动脉血管内皮脱落、内膜增殖及外膜沉积等病理改变,整体改善大鼠主动脉组织病理损伤。表明芍药苷对SSH大鼠血管内皮功能起到保护作用。
PI3K/AKT/mTOR信号通路参与生命过程中的多种生物功能。激活的PI3K/AKT信号通路对平滑肌细胞和血管成纤维细胞的分化、增殖和凋亡有重要影响[22-23]。mTOR作为PI3K/AKT信号通路的下游元件,PI3K/AKT信号通路激活会促进mTOR磷酸化激活。PI3K/AKT/mTOR信号通路是调节细胞生存、增殖、生长和运动等多种正常过程的典型信号通路[24],对该通路的深入研究表明,PI3K/AKT/mTOR信号通路在高血压调控中发挥重要作用[25]。已有研究报道,芍药苷可通过调控PI3K/AKT/mTOR信号通路发挥抗炎和免疫调节作用[26]。本研究中,芍药苷显着降低SSH大鼠主动脉组织中PI3K、AKT和mTOR磷酸化水平,抑制PI3K/AKT/mTOR信号通路激活,并且这种抑制作用被PI3K/AKT/mTOR信号通路激活剂740Y-P减弱。此外,740Y-P显着减弱了芍药苷对SSH大鼠血压、血管内皮功能的改善作用,但740Y-P没有完全逆转芍药苷对SSH的作用效果。因此,芍药苷可能部分通过抑制PI3K/AKT/mTOR信号通路活化降低SSH大鼠血压、改善大鼠血管内皮功能。
综上所述,芍药苷可以有效降低SSH大鼠血压,并改善大鼠血管内皮功能。芍药苷介导的抗高血压和保护血管内皮功能作用可能部分与抑制PI3K/AKT/mTOR信号途径激活有关。本研究为芍药苷在SSH中的作用效果提供了实验证据,芍药苷对SSH具有潜在治疗作用。未来的研究需要阐明芍药苷介导的SSH中PI3K/AKT/mTOR途径抑制的确切潜在机制以及芍药苷可能影响的其他信号转导途径。
参考文献:
[1]STEPHANIE M M,ANNET K,THOMAS K R.Epithelial sodium channel and salt-sensitive hypertension[J].Hypertension,2021,77(3):759-767.
[2]RUST P,EKMEKCIOGLU C.Impact of salt intake on the pathogenesis and treatment of hypertension[J].Adv Exp Med Biol,2017,956:61-84.
[3]LIN X,HAN T,FAN Y,et al.Quercetin improves vascular endothelial function through promotion of autophagy in hypertensive rats[J].Life Sci,2020,258:118106-118120.
[4]KONUKONLU D,UZUN H.Endothelial dysfunction and hypertension[J].Adv Exp Med Biol,2017,956:511-540.
[5]JIAO F,VARGHESE K,WANG S X,et al.Recent insights into the protective mechanisms of paeoniflorin in neurological,cardiovascular,and renal diseases[J].Journal of Cardiovascular Pharmacology,2021,77(6):728-734.
[6]CHEN C,DU P,WANG J.Paeoniflorin ameliorates acute myocardial infarction of rats by inhibiting inflammation and inducible nitric oxide synthase signaling pathways[J].Mol Med Rep,2016,12(3):3937-3943.
[7]LIU X,CHEN K,ZHUANG Y,et al.Paeoniflorin improves pressure overload-induced cardiac remodeling by modulating the MAPK signaling pathway in spontaneously hypertensive rats[J].Biomed Pharmacother,2019,111:695-704.
[8]ZHAI J,GUO Y.Paeoniflorin attenuates cardiac dysfunction in endotoxemic mice via the inhibition of nuclear factor-κB[J].Biomed Pharmacother,2016,80:200-206.
[9]漆秦,王显利,吴朝,等.法舒地尔对盐敏感性高血压大鼠心室重构及心肌细胞凋亡的影响研究[J].实用心脑肺血管病杂志,2017,25(12):49-54.
[10]王贞丽,姜萍,李丙华,等.芍药苷对大鼠低氧性肺动脉高压的防治作用及其抗氧化机制研究[J].中国海洋药物,2022,41(4):51-58.
[11]LI B,YANG Z B,LEI S S,et al.Beneficial effects of paeoniflorin enriched extract on blood pressure variability and target organ damage in spontaneously hypertensive rats[J].Evid Based Complement Alternat Med,2017,2017:5816960-5816981.
[12]LI B,YANG Z B,LEI S S,et al.Combined antihypertensive effect of paeoniflorin enriched extract and metoprolol in spontaneously hypertensive rats[J].Pharmacogn Mag,2018,14(53):44-52.
[13]WU J,ZHANG D,HU L,et al.Paeoniflorin alleviates NG-nitro-L-arginine methyl ester(L-NAME)-induced gestational hypertension and upregulates silent information regulator 2 related enzyme 1(SIRT1)to reduce H2O2-induced endothelial cell damage[J].Bioengineered,2022,13(2):2248-2258.
[14]YU M,PENG L Y,LIU P,et al.Paeoniflorin ameliorates chronic hypoxia/SU5416-induced pulmonary arterial hypertension by inhibiting endothelial-to-mesenchymal transition[J].Drug Design,Development and Therapy,2020,14:1191-1202.
[15]YU M,WU X C,WANG J J,et al.Paeoniflorin attenuates monocrotaline-induced pulmonary arterial hypertension in rats by suppressing TAK1-MAPK/NF-κB pathways[J].International Journal of Medical Sciences,2022,19(4):681-694.
[16]CAI R,HAO Y,LIU Y Y,et al.Tumor necrosis factor alpha deficiency improves endothelial function and cardiovascular injury in deoxycorticosterone acetate/salt-hypertensive mice[J].Biomed Res Int,2020,2020:3921074-3921089.
[17]LIANG K L,FANG P,SHI Q Q,et al.Antihypertensive effect and mechanism of Dendrobium officinale flos on high-blood pressure rats induced by high glucose and high fat compound alcohol[J].China Journal of Chinese Materia Medica,2018,43(1):147-153.
[18]XIE P,CUI L,SHAN Y,et al.Antithrombotic effect and mechanism of radix paeoniae rubra[J].Biomed Res Int,2017,2017:9475074-9475083.
[19]ZHANG J,HUA W,ZHAO X,et al.Paeoniflorin alleviates endothelial dysfunction caused by overexpression of soluble fms-like tyrosine kinase 1 and soluble endoglin in preeclampsia via VEGFA upregulation[J].Biosci Biotechnol Biochem,2021,85(4):814-823.
[20]WANG J S,HUANG Y,ZHANG S,et al.A protective role of paeoniflorin in fluctuant hyperglycemia-induced vascular endothelial injuries through antioxidative and anti-inflammatory effects and reduction of PKCβ1[J].Oxid Med Cell Longev,2019,2019:5647219.
[21]KONG L C,LI J J,YANG Y Q,et al.Paeoniflorin alleviates the progression of retinal vein occlusion via inhibiting hypoxia inducible factor-1α/vascular endothelial growth factor/STAT3 pathway[J].Bioengineered,2022,13(5):13622-13631.
[22]HUANG C,FANG X,XIE X,et al.Effect of miR-126 on the proliferation and migration of vascular smooth muscle cells in aortic aneurysm mice under PI3K/AKT/mTOR signaling pathway[J].Mol Biotechnol,2021,63(7):631-637.
[23]SUN F,BI Q,WANG X,et al.Down-regulation of mir-27b promotes angiogenesis and fibroblast activation through activating PI3K/AKT signaling pathway[J].Wound Repair Regen,2020,28(1):39-48.
[24]ORTEGA MIGUEL A,NGEL A,JAVIER L,et al.Implication of the PI3K/AKT/mTOR pathway in the process of incompetent valves in patients with chronic venous insufficiency and the relationship with aging[J].Oxidative Medicine and Cellular Longevity,2018,2018:1495170.
[25]PAN L,SUN X,CHE H,et al.CTRP9 mitigates vascular endothelial cell injury in patients with hypertensive heart disease by inhibiting PI3K/Akt/mTOR axis[J].Am J Transl Res,2022,14(9):6596-6603.
[26]ZHANG L L,WEI W.Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony[J].Pharmacology & Therapeutics,2020,207:107452.
(收稿日期:2022-12-01)
(本文编辑王雅洁)



