靳莎莎,庞水秀,赵利娜,张红印,*,张晓云,束兆林
(1.江苏大学食品与生物工程学院,江苏 镇江 212013;2.江苏丘陵地区镇江农业科学研究所,江苏 镇江 212013)
Postharvest diseases limit the storage period and marketing life of fruits. Disease control is achieved mainly through the use of fungicides, benomyl and iprodione were available for postharvest treatment to reduce decay and to extend the shelf-life of stone fruits[1]. However, a growing international concern over the often indiscriminate use of synthetic fungicides on food crops because of the potential harmful effects on human health[2]. In recent years, consumers are demanding less chemical residue on produce, and many fungi are developing resistance to commonly used fungicides[3]. The use of fungicides is becoming more restricted due to health concerns[4]. Therefore, it is necessary to develop alternatives to synthetic chemical control to reduce environmental risks and raise consumer confidence.
Biological control has emerged as an effective strategy to combat major postharvest decays of fruits[5]. The first step in developing biocontrol agents is the isolation and identification process which will largely influence the efficacy and its ultimate success under commercial conditions. Wilson et al.[6]noted that special consideration was needed in identifying the potential antagonists. For instance, Wilson et al.[7]reported their strategy to utilize fruit wounds to screen for the potential yeast antagonists against postharvest rot organisms from unidentified microbial populations on fruit surfaces. This strategy allows for the rapid selection of a number of the potential antagonists for the control of postharvest diseases of fruits with a minimal expenditure of time and expense, and has been used in many postharvest biocontrol programs throughout the world.
After getting the potential antagonist yeast, it must be identified. The biochemical methods and the molecular methods are usually used to identify the potential antagonist yeast isolated. The most relevant molecular method used in the identification of yeast species are based on the variability of the ribosomal genes 5.8S, 18S and 26S[8-11]. The interest in ribosomal genes for species identification comes from the concerted fashion in which they evolve showing a low intraspecific polymorphism and a high interspecific variability[8]. Previous results have demonstrated that the complex ITS regions (non-coding and variable) and the 5.8S rRNA gene (coding and conserved) are useful in measuring close fungus phylogenetic relationships since they exhibit far greater interspecific differences than the 18S and 26S rRNA genes[9-11]. The biochemical methods were usually used to confirm identification.
The objectives of this study were to evaluate: 1) isolation a strain of potential antagonistic yeast from the soil samples of unsprayed orchards, and then molecular method and biochemical method were used to identify the organism which showed the best control efficacy; 2) the efficacy of the antagonist yeast for the biocontrol of gray mold decay and Rhizopus decay of strawberries; 3) the population growth of the antagonist yeast in strawberries wounds; 4) the efficacy of the antagonist yeast in controlling of natural decay development of strawberries; 5) its effects on quality parameters of strawberries after storage, including firmness, total soluble solids, ascorbic acid and titratable acidity.
1 Materials and Methods
1.1 Fruit
Pear fruits (Pyrus pyrifolia Nakai.) cultivars “Fengshui” were harvested at commercial maturity from Zhenjiang Jiangsu province. Fruits were used immediately after harvest or after less than 3 months of storage at 1 ℃. Fruits were washed with tap water after disinfected with 0.1% sodium hypochlorite for 2 min, and then were air dried prior to wounding.
Strawberries (Fragaria ananassa Duch.) cultivars “Fengxiang” were harvested early in the morning from the orchard and rapidly transferred to the laboratory. Berries were sorted on the basis of size, color (75% full red color) and absence of physical damage, and were randomLy divided into lots of twenty fruit.
1.2 Pathogen inoculum
Botrytis cinerea which was used to test the biocontrol efficacy of isolated organisms to gray mold decay of pears was isolated from infected pears. Botrytis cinerea and Rhizopus stolonifer which were used to test the biocontrol efficacy of the antagonist chosen for controlling of gray mold decay or Rhizopus decay of strawberries were isolated from infected strawberries. The cultures were maintained on potato-dextrose agar medium (PDA: extract of boiled potatoes, 200 mL; dextrose, 20 g; agar, 20 g and distilled water, 800 mL) at 4 ℃, and fresh cultures were grown on PDA plates before use, respectively. Spore suspensions were prepared by removing the spores from the sporulating edges of a 7 d old culture with a bacteriological loop, and suspending them in sterile distilled water. Spore concentrations were determined with a hemocytometer, and adjusted as required with sterile distilled water.
1.3 Antagonist
The antagonist yeast isolates were maintained at 4 ℃ on Nutrient Yeast Dextrose Agar (NYDA) medium containing 8 g nutrient broth, 5 g yeast extract, 10 g glucose and 20 g agar (Sangon Co., Shanghai, China), in 1 L of distilled water. Liquid cultures of the yeast were grown in 250 mL Erlenmeyer flasks containing 50 mL of NYD Broth (NYDB) which had been inoculated with a loop of the culture. Flasks were incubated on a rotary shaker at 28 ℃ for 24 h. Following incubation, cells were centrifuged (TGL-16M Centrifuge, Xiangyi Co., Changsha, China) at 5000×g for 10 min and washed twice with sterile distilled water in order to remove the growth medium. Yeast cell pellets were resuspended in sterile distilled water and adjusted to an initial concentration of approximately 1×109cells/mL before adjusted to 1×108, 1×107cells/mL and 1×106cells/mL for the different experiments.
1.4 Samples selection and isolation of the organisms
The samples: soil under the fruit trees, which were collected from an organic orchard of south east of Zhenjiang, Jiangsu Province. They were conveyed to the laboratory for analysis as soon as possible.
One gram of the soil sample was mixed with sterile distilled water. The quantity of soil sample was diluted 10 times, 102times, and 103times respectively in 10 mL tubes with sterile water. A 100 μL of each suspension was shown on the surface of NYDA plate. Plates were incubated at 28 ℃ for 2 d. The forming colonies were chosen. They were maintained at 4 ℃ on NYDA.
1.5 Test of biocontrol efficacy of organisms isolated
Cell concentrations of organisms isolated were then adjusted to 1×108cells/mL with sterile distilled water by a hemocytometer, respectively, and sterile distilled water acted as the control. The surface of pears was wounded with a sterile cork borer (approximately 3-mm-diameter and 3-mmdeep). Washed cell suspensions of organisms isolated were added into each wound, respectively. Three hours later, 15 μL of 1×105spores/mL suspension of B. cinerea was inoculated to each wound respectively. After air drying, the samples were stored in enclosed plastic trays to maintain a high relative humidity (about 95%) and incubated at 25 ℃. The percentage of infected fruit was recorded after 3 d inoculation. There were three replicates of 20 fruits for each treatment, and the experiment was conducted twice.
1.6 Organism identification
Based of the research results of “test of biocontrol efficacy of organisms isolated”, the organism that showed the best control efficacy was identified. The organism identification methods were as followed.
1.6.1 Molecular identification
The strain was preliminarily characterized by means of molecular identification based on comparative sequence analysis of 5.8S rDNA gene. The strain was incubated at 28 ℃ for 20 h prior to the PCR reaction. The PCR reaction (25 μL) mixture contained 4 μL of 10×reaction buffer, 2 μL dNTP mix (2 mmol/L), 2 μL ITS1 primer (5’-TCCGTAGGTGAACCTGCGG-3’), 2 μL ITS2 primer (5’-TCCTCCGCTTATTGATATGC-3’), 1.6 μL MgCl2(25 mmol/L), 2 μL suspensions of cells and 10.4 μL deionized H2O. The PCR was performed on an automated thermocycler device using the following parameter: Adding 1 μL Taq DNA polymerase (5 U/μL) after denaturation at 94 ℃ for 4 min followed by 30 cycles of denaturation at 94 ℃ for 50 s, annealing at 52 ℃ for 50 s, elongating at 72 ℃ for 60 s and a final extension at 72 ℃ for 6 min.
The PCR products were detected by gel electrophoresis and sent to Shanghai Sangon Biological Engineering Technology and Services Co. Ltd., to sequence. The 5.8S rDNA sequence of strain was analyzed and aligned with the related sequences retrieved from GenBank database using NCBI BLAST searching tool. A phylogenetic tree based on 5.8S rDNA sequences was constructed with the Neighbor-Joining algorithm in MEGA version 2.1.
1.6.2 Biochemical methods
The strain was further identified based on biochemical and morphology characters. Assimilation studies of glucose, sucrose, lactose, maltose, trehalose, mannitol, citric acid, and starch were carried out on bean juice agar medium. Sporulation was determined on McClary medium[12]. Cellular morphology was observed under the microscope after growth for 48 h in NYDB at 28 ℃. And the colony morphology was observed after growing for 48 h in NYDA at 28 ℃.
1.7 Effect of the antagonist on controlling of gray mold decay and Rhizopus decay of strawberries
The surface of strawberries was wounded with a sterile cork borer (approximately 3 mm-diameter and 3 mmdeep). The suspensions of washed cells were adjusted to the concentrations of 1×106, 1×107, 1×108, 1×109cells/mL with sterile distilled water by a hemocytometer, respectively. Each wound was treated with 30 μL of the cell suspensions of P. caribbica at 1×106, 1×107, 1×108, 1×109cells/mL and sterile distilled water as the control. Three hours later, 15 μL of 1×105spores/mL suspension of B.cinerea, or 5×104spores/mL suspension of R.stolonifer was inoculated into each wound respectively. After air drying, the samples were stored in enclosed plastic trays to maintain a high relative humidity (about 95%) and incubated at 20 ℃. The percentage of infected fruit was recorded after 3 d inoculation. There were three replicates of 20 fruits for each treatment, and the experiment was conducted twice.
1.8 Population growth of the antagonist in strawberry wounds
Fruit samples were wounded as described above to evaluate the biocontrol to gray mold decay and Rhizopus decay of strawberries. The wounds were treated with 30 μL of a suspension of P. caribbica at 1×108cells/mL. The samples were taken at 0, 1, 2 d and 3 d at 20 ℃ or at 0, 5, 10, 15 d and 20 d at 4 ℃ after treatment. The tissue was removed with a sterile cork borer (9 mm-diameter and 10 mm-deep) and ground with a 150 mL Erlenmeyer flasks, by glass rod directly pounding to pieces in 50 mL of sterile 0.85% sodium chloride solution. Serial 10-fold suitable dilutions were made and 0.1 mL of suitable dilution was spread in NYDA. The plates were incubated at 28 ℃ for 2 d, and the colonies were counted. Population densities of P. caribbica were expressed as lg (CFU/wound). There were three replicates per treatment and 6 fruits per replicate, and the experiments were repeated twice[13].
1.9 Effect of the antagonist on natural decay development and quality parameters of strawberries
The suspensions of washed cells of organism were adjusted to the concentrations of 1×108cells/mL with sterile distilled water by a hemocytometer, and sterile distilled water as the control. Intact fruit were inoculated by dipping them in the suspension of washed cells or sterile distilled water respectively for 30 s, and air-dried. The treated fruits were sealed in polyethylene-lined plastic boxes to retain high humidity (about 95%). Treated fruits were stored at 20 ℃ for 7 d in order to determine disease development under normal shelf-life conditions. The percentage of infected fruits was recorded afterwards. There were three replicate trials of 20 fruits per treatment with a complete randomization. The experiments were repeated three times.
To evaluate the effect of antagonistic organism on postharvest quality of strawberries, freshly harvested fruits were treated and stored as described above to evaluate the effect of antagonist on reducing natural decay development of strawberries. Quality parameters were measured after storage, on three replicates of five fruits each, and performed at ambient temperature (about 20 ℃). The testing methods are described below.
1.9.1 Fruit firmness
Firmness values of each individual strawberry were measured at three points of the equatorial region by using the TA-XT2i Texture Analyser (Microstable Instruments, UK) with a 5 mm diameter flat probe. The probe descended toward the sample at 5.0 mm/s and the maximum force (N) was defined as firmness. The firmness of each strawberry was measured three times on different sides.
1.9.2 Total soluble solids
Total soluble solids (TSS) were determined by measuring the refractive index of the same juice with a hand refractometer and the results expressed as percentages (g/100 g fruit weight)[14].
1.9.3 Ascorbic acid
The 2,6-dichloroindophenol titrimetric method[15]was used to determine the ascorbic acid content of pressed fruit juice. Results were expressed as milligrams of ascorbic acid per 100 g sample[16].
1.9.4 Titratable acidity
Acidity was measured by titration with 0.1 mol/L NaOH to pH 8.1; 4 g of juice diluted with 20 mL of distilled water was evaluated for each replicate. Titratable acidity was calculated as percent malic acid[17].
2 Results and Analysis
2.1 Isolation of organisms
After plates were incubated at 28 ℃ for 2 d, there were many similar colony form units, which are cream, round and opaque. Four colonies were picked out. They were maintained at 4 ℃ on NYDA for tests the biocontrol efficacy. Strain NO.4 showed the highest activity in controlling gray mold decay of pears, and the infection percentage of pears treated with strain NO.4 was significantly lower than that treated with other strains and the control (Table 1).

Table 1 Efficacy of four isolated organisms in controlling the gray mold decay of pears
2.2 Identification of the potential organism
2.2.1 Molecular identification
The result of sequencing of the active strain NO.4 was obtained in form of rough electrophoregramms under Chromas software. The alignments of the sequence were carried out by Chromas Lite software. The obtained sequence was subjected via internet using the BLAST software, for comparison with the homologous sequences contained in the genebank database (accession number: lcl|50765). The analysis of 5.8S rDNA sequence indicated that the strain shared 99% maximal identity with Pichia caribbica. Moreover, strain NO.4 clustered with several strains from GenBank in the phylogenetic tree (Fig.1), clearly demonstrating that the strain is a member of P.caribbica at 5.8S rDNA sequence homology level.
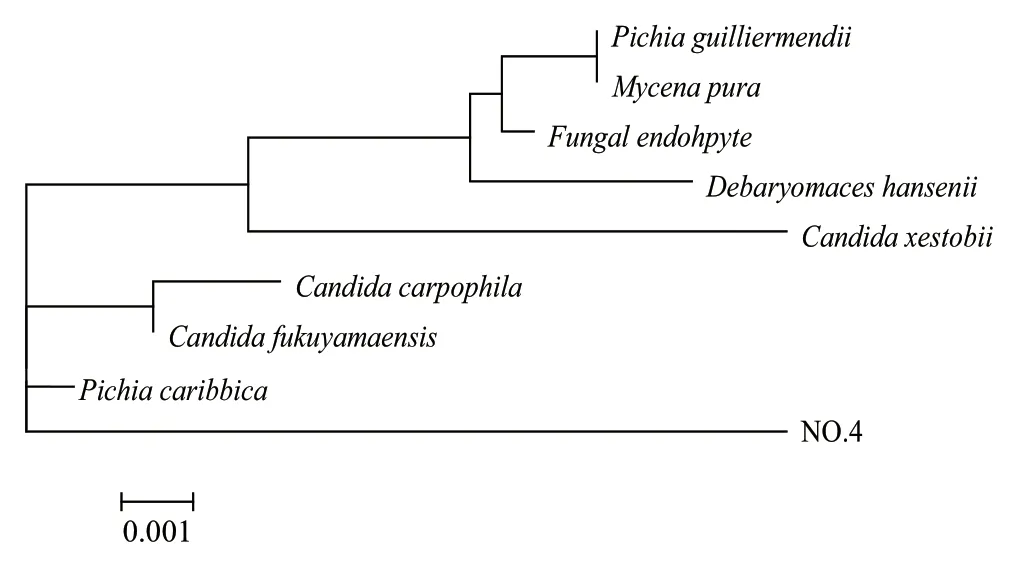
Fig.1 Neighbor-Joining phylogenetic tree based on 5.8S rDNA sequences showing the relationships between strain NO.4 and related strains from GenBank database
2.2.2 Biochemical methods
The colony color of strain NO.4 is cream. The colony appearance is smooth. Cell shape is oval. Ascospores were observed after 4 d at 28 ℃ on spore medium. Assimilation situation of strain NO.4 is as following (Table 2). According to physiological characteristics described by CBS (Fungal Biodiversity Centre), strain NO.4 was confirmed to be P. caribbica.
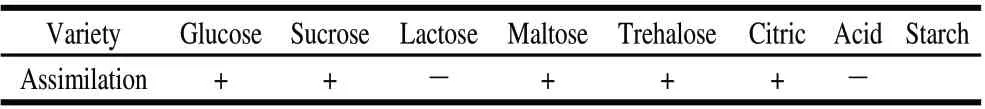
Table 2 Assimilation situation of strain NO.4
2.3 Effect of the antagonist on controlling of gray mold decay and Rhizopus decay of strawberries
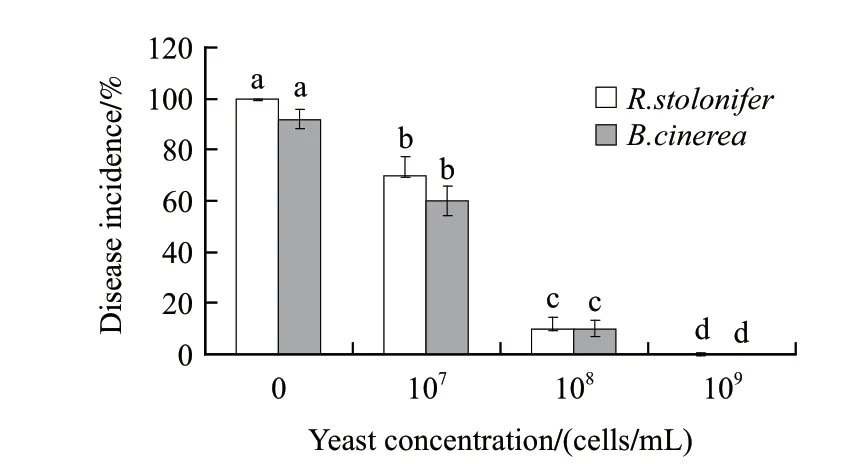
Fig.2 Efficacy of the antagonist in controlling the gray mold decay and Rhizopus decay of strawberries
The antagonist(P. caribbica)showed high activity in controlling gray mold decay and Rhizopus decay of strawberries (Fig.2). The concentrations of antagonist significantly influenced disease incidence of gray mold decay and Rhizopus decay of strawberries. The results showed that the higher the concentrations of the antagonist, the lower the disease incidence. The concentrations of the antagonist at 1×109cells/mL, the gray mold decay and Rhizopus decay of strawberries were completely inhibited after 3 d incubation at 20 ℃, while the control fruits had 92.5% decay incidence caused by B.cinerea, and 100% decay incidence caused by R. stolonifer, respectively.
2.4 Population growth of the antagonist in strawberries wounds
Rapid growth of the antagonist in strawberries wounds was observed during the first day at 20 ℃ when the antagonist was applied to wounds, and then the populations stabilized for the remaining storage period (Fig.3A). Similarly, on strawberry wounds kept at 4 ℃, rapid growth of the yeast in wounds was observed during the first 5 d after being applied, and then the populations stabilized for the remaining storage period (Fig.3B). Repeated trials gave similar results.
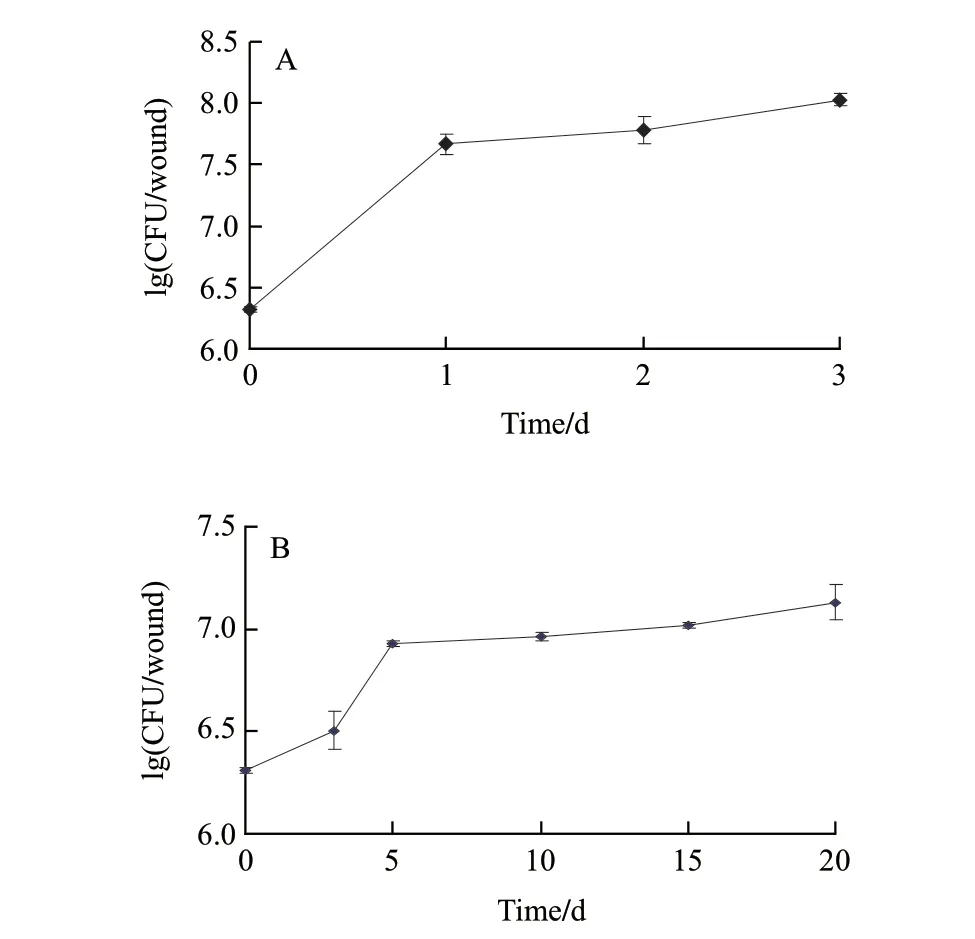
Fig.3 Population growth of the antagonist in wounds of strawberries at 20 ℃ (A) or 4 ℃ (B)
2.5 Effect of the antagonist on natural decay development and quality parameters of strawberries
Our experiments evaluated the efficacy of yeast antagonist in reducing the natural development of decay following storage at 20 ℃ for 5 d. The results presented in Table 3 indicated that the application of the antagonist resulted in low average decay incidence in fruit at 46.33%, compared with 76.25% in the water-treated control fruit. The yeast antagonist had no significant effect on soluble solids, ascorbic acid or titratable acidity after 5 d at 20℃ (Table 3). Fruit firmness treated with antagonist yeast was significantly higher than that of the control fruits.
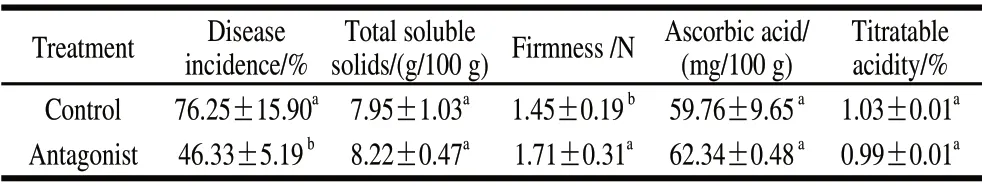
Table 3 Effect of P.caribbica on the natural decay and quality of strawberries
3 Discussion
In this study, we isolated and identified a strain of potential yeast antagonist against postharvest decay of fruits from unidentified microbial populations in the soil sample of unsprayed orchards. As it is shown in this paper, the use of molecular method and biochemical method has allowed the identification of the potential antagonist yeast isolated. After compared with the known species in the NCBI, the potential yeast antagonist isolation has the closest strain match with P. caribbica. Biochemical method confirmed that the potential yeast antagonist is P. caribbica. To the best of our knowledge, P. caribbica has not been described previously in biocontrol of postharvest diseases of fruits.
When wounds on strawberries fruits were treated with the antagonist yeast, the developments of lesions caused by B.cinerea and R. stolonifer were effectively reduced or prevented. This showed that the antagonist yeast has potential as a biocontrol agent for the control of postharvest gray mold decay and Rhizopus decay of fruits. Gray mold decay and Rhizopus decay caused by B.cinerea and R.stolonifer are two important postharvest diseases of strawberries. P.caribbica having a control efficacy to B.cinerea and R.stolonifer indicated that it may control natural development of decay of strawberries mainly caused by these pathogens.
We also investigated the population growth of P.caribbica in fruits wounds at 20 ℃ and at 4 ℃. Our result showed that the antagonist could rapidly colonize the fruit tissues at the first day at 20 ℃, and at the first five day at 4 ℃, and then the populations stabilized for the remaining storage period. Even after a period of 20 d at 4 ℃, the number of viable microorganisms was similar to or greater than that originally introduced into the wounds. These show that the nutritional environment at the wound site may be favorable to the antagonist, which rapidly colonizes the fruit tissues and will be competing with the pathogens for nutrients. The biocontrol yeasts have been selected mainly for their capacity to rapidly colonize and multiply in surface wounds, and subsequently to compete with the pathogen for nutrients and space[18].
Many results suggested that nutrient competition plays a major role in the biocontrol of many postharvest antagonists[19-20]. The demonstrated effect of initial concentration of the antagonist on biocontrol efficacy and rapid colonization in fruit wounds provide presumptive evidence that biocontrol is achieved when actively multiplying populations of the antagonist are present in wounds[21].
P. caribbica significantly reduced the natural development of decay of strawberries following storage at 20 ℃ for 5 d. These also suggested that P.caribbica has a control efficacy at a wide range of pathogens. In addition, the antagonist did not impair quality parameters of strawberries under laboratory conditions. All these suggested that P. caribbica has potential for commercialization.
Wilson et al.[7]indicated the following characteristics of an ideal antagonist: genetic stability, efficacy at low concentrations and against a wide range of pathogens on various fruit products, simple nutritional requirements, survival in adverse environmental conditions, growth on cheap substrates in fermenters, lack of pathogenicity for the host plant and no production of metabolites potentially toxic to humans, resistance to the most frequently used pesticides and compatibility with other chemical and physical treatments. P. caribbica originally survive in such a harsh environment and have a large antagonist population. It seems to possess a good number of the above-mentioned features, and has the potential as an ideal antagonist.
In conclusion, P. caribbica isolated from the soil sample of unsprayed orchards showed significant control efficacy to postharvest gray mold decay, Rhizopus decay and natural decay of strawberries. It is the first report that P. caribbica can be an antagonist to fungi of fruits. Further research will be aimed at investigating it’s efficacy for controlling of other pathogens from more fruits, the possible mechanisms could study to reveal the mode of action of the antagonist yeast, and enhancing it’s efficacy by combining with other approach.
[1] FAN Q, TIAN S P, LI Y X, et al. Biological control of postharvest brown rot in peach and nectarine fruits by Bacillus subtilis (B-912)[J]. Acta Bot Sin, 2000, 42: 1137-1143.
[2] NORMAN C. EPA sets new policy on pesticide cancer risks[J]. Science, 1988, 242: 366-367.
[3] CONWAY W S, LEVERENTZ B, JANISIEWICZ W J, et al. Integrating heat treatment, biocontrol and sodium bicarbonate to reduce postharvest decay of apple caused by Colletotrichum acutatum and Penicillium expansum[J]. Postharvest Biol Tec, 2004, 34: 11-20.
[4] RAGSDALE N N, SISLER H D. Social and political implications of managing plant diseases with decreased availability of fungicides in the United States[J]. Annu Rev Phytopathol, 1994, 32: 545-557.
[5] JANISIEWICZ W J, KORSTEN L. Biological control of postharvest diseases of fruits[J]. Annu Rev Phytopathol, 2002, 40: 411-441.
[6] WILSON C L, WISNIEWSKI M. Biological control of postharvest diseases of fruits and vegetables: an emerging technology[J]. Annu Rev Phytopathol, 1989, 27: 425-441.
[7] WILSON C L, WISNIEWSKI M, DROBY S, et al. A selection strategy formicrobial antagonist to control postharvest diseases of fruits and vegetables[J]. Hortic Sci, 1993, 53: 183-189.
[8] LI W H. Molecular evolution[M]. Sunderland, MA: Sinauer Associates, 1997.
[9] CAI J, ROBERTS I N, COLLINS M D. Phylogenetic relationshipsamong members of the ascomycetous yeasts genera Brettanomyces, Debaromyces, Dekkeraand kluyveromyces deduced by small-subunit rRNA gene sequences[J]. Int J Syst Bacteriol, 1996, 46: 542-549.
[10] JAMES S A, COLLINS M D, ROBERTS I N. Use of an rRNA internal transcribed spacer region to distinguish phylogenetically closely related species of the genera Zygosaccharomyces and Torulaspora[J]. Int J Syst Bacteriol, 1996, 46: 189-194.
[11] KURTZMAN C P. rRNA sequence comparisons for assessing phylogenetic relationships among yeasts[J]. Int J Syst Bacteriol, 1992, 42: 1-6.
[12] McCLARY D O, NULTY W L, MILLER G R. Effect of potassium versus sodium in the sporulation of Saccharomyces[J]. J Bacteriol, 1959, 78: 362-368.
[13] ZHANG H Y, WANG L, DONG Y, et al. Postharvest biological control of gray mold decay of strawberry with Rhodotorula glutinis[J]. Biol Control, 2007, 40: 287-292.
[14] LARRIGAUDIÈRE C, PONS J, TORRES R, et al. Storage performance of clementines treated with hot water, sodium carbonate and sodium bicarbonate dips[J]. J Hortic Sci Biotech, 2002, 77: 314-319.
[15] AOAC Official methods of analysis[M]. 16th ed. 45.1.14. AOAC, Arlington, Virginia. 1995.
[16] ÖZDEN Ç, BAYINDIRLI L. Effects of combinational use of controlled atmosphere, cold storage and edible coating applications on shelf life and quality attributes of green peppers[J]. Eur Food Res Technol, 2002, 214: 320-326.
[17] WRIGHT K P, KADER A A. Effect of controlled-atmosphere storage on the quality and carotenoid content of sliced persimmons and peaches[J]. Postharvest Biol Tec, 1997, 10: 89-97.
[18] DROBY S, VINOKUR V, WEISS B, et al. Induction of resistance to Penicillium digitatum in grapefruit by the yeast biocontrol agent Candida oleophila[J]. Phytopathology, 2002, 92: 393-399.
[19] ZHANG H Y, ZHENG X D, XI Y F. Biological control of postharvest blue mold of oranges by Cryptococcus laurentii (Kufferath) Skinner[J]. Biol Control, 2005, 50: 331-342.
[20] DROBY S, WISNIEWSKI M, DUMITRU M, et al. Twenty years of postharvest biocontrol research: is it time for a new paradigm?[J]. Postharvest Biol Tec, 2009, 52: 137-145.
[21] ZHENG X D, ZHANG H Y, SUN P. Biological control of postharvest green mold decay of oranges by Rhodotorula glutinis[J]. Eur Food Res Technol, 2005, 220: 353-357.



